
- HOME |
- ABOUT THE CENTER |
- RESEARCH |
- PEOPLE |
- PODCAST |
- NEWS |
- CONTACT US
Our Research
The Center for Lignocellulose Structure and Formation (CLSF) is focused on developing a detailed understanding of lignocellulose, the main structural material in plants.
Perspective
Every living organism on Earth uses glucose as an energy source. Plants not only make glucose from sunlight, water and carbon dioxide, but they convert much of it into an energy-rich material – the lignocellulosic cell wall – that is both a versatile material and a recalcitrant feedstock for liquid biofuel production, both properties stemming from its hierarchical structure at the nano- to mesoscales. Our research addresses key questions in plant biology: by understanding the fundamental science of how plants manufacture lignocellulose, we may devise new ways to control it (through genetic engineering) and transform it (through chemical engineering) for improved technologies to supply our energy and material needs for a sustainable future.
Research Plan and Direction
CLSF researchers investigate:
- how plants transform glucose into the crystalline nano-fibril (cellulose) with very different physical properties from the starting material
- how cellulose is combined with other polymers to make cell walls with diverse physical properties
These manufacturing processes, practiced by nearly every plant cell, greatly exceed the current capabilities of human technologies. The goals of the CLSF are to develop a detailed nano- to meso-scale understanding of plant cell walls, from glucose polymerization and glucan crystallization into cellulose microfibrils to the orderly hierarchical assembly of the components to form the mature plant cell wall. Success in our research goals will offer many lessons in how to create such hierarchical structures, how to vary and manipulate them, and potentially how to disassemble them more efficiently than is currently possible. Our team of accomplished biological, physical and computational researchers with their diverse technical expertise and team collaboration work towards these goals which straddle biology and physics.
Research Highlights
- “Most polysaccharide biosynthesis and some modifications, including methylation, occur in the Golgi body. Thus, identifying potential transporters of SAM into the Golgi is important for understanding the role of pectin modifications in cell wall structure and function.” The editorial team of Nature Plants provided this statement in their research briefing “Shutting the door on polysaccharide methylation" which highlighted CLSF’s latest research publication Golgi-localized putative S-Adenosyl methionine transporters required for plant cell wall polysaccharide methylation Temple et al. 2022. (June 2022)
- A CLSF collaborative team used an innovative combination of cryo-electron tomography and sensitivity-enhanced solid-state NMR to detail the structure of cellulose fibers synthesized in-vitro by CESAs present in membrane fractions of Physcomitrium patens (a moss) in Deligey, Frank et al. 2022. The combination of these techniques allows us to detail both the nanoscale assembly and atomic-level structure of cellulose fibers. The work is published in Biomacromolecules with a highlight as a supplementary journal cover. (April 2022)
- Lignin structure and xylan conformation regulate the physical packing interactions of lignin and polysaccharides and have variable patterns among grasses, hardwoods, and softwoods. These findings by Kirui et al. utilizing solid-state NMR, unveil the principles of polymer interactions underlying the heterogeneous architecture of lignocellulosic materials. The article is published in Nature Communications and is selected by the editors as a featured article within the topic of Structural Biology, Biochemistry and Biophysics. (January 27, 2022)
 The results of altering the protein sequence of a CESA gating loop, which is predicted to help regulate cellulose synthase enzyme activity, is reported in Burris et al. 2021. Seven changes were tested in a secondary wall cellulose synthase and compared to prior data on parallel changes in two other divergent cellulose synthase isomers involved in primary wall synthesis. The results demonstrate the potential of protein engineering to modulate cellulose synthesis through subtle changes in regulatory regions. (August 2021)
The results of altering the protein sequence of a CESA gating loop, which is predicted to help regulate cellulose synthase enzyme activity, is reported in Burris et al. 2021. Seven changes were tested in a secondary wall cellulose synthase and compared to prior data on parallel changes in two other divergent cellulose synthase isomers involved in primary wall synthesis. The results demonstrate the potential of protein engineering to modulate cellulose synthesis through subtle changes in regulatory regions. (August 2021)- A plant cell wall’s unique ability to expand without weakening or breaking—a quality required for plant growth—is due the movement of its cellulose skeleton, according to new research that models the cell wall. The new model, created by Penn State CLSF researchers, reveals that chains of cellulose bundle together within the cell wall, providing strength, and slide against each other when the cell is stretched, providing extensibility. The new study, which appears online May 14 in the journal Science, presents a new concept of the plant cell wall, gives insights into plant cell growth, and could provide inspiration for the design of polymeric materials with new properties. Read PSU news coverage and watch a short video that demostrates the dynamics during stretching.
 Research led by Enrique Gomez and Esther Gomez at Penn State has identified, for the first time, that cellulose crystals have a preferred orientation relative to the cell wall in plants and may be due to some common consequence of how plants make their cell walls. These findings published in September in Nature Communications may help settle a long-standing debate in the cellulose field — whether crystals within plant cell walls twist — because heaving a preferred orientation suggests that crystals aren't twisting. These findings came as a result of applying a technique called grazing-incidence wide-angle X-ray scattering (GIWAXS) “developed for materials science and used extensively for the study of thin films, including polymer films” to the study of plant cell walls. Read PSU news coverage or open access research article in Nature Communications.
Research led by Enrique Gomez and Esther Gomez at Penn State has identified, for the first time, that cellulose crystals have a preferred orientation relative to the cell wall in plants and may be due to some common consequence of how plants make their cell walls. These findings published in September in Nature Communications may help settle a long-standing debate in the cellulose field — whether crystals within plant cell walls twist — because heaving a preferred orientation suggests that crystals aren't twisting. These findings came as a result of applying a technique called grazing-incidence wide-angle X-ray scattering (GIWAXS) “developed for materials science and used extensively for the study of thin films, including polymer films” to the study of plant cell walls. Read PSU news coverage or open access research article in Nature Communications.- CLSF researchers determined the structure of a cellulose synthase CesA homotrimer which enables structural insights into the unique nanomachinery used by plants to form cellulose chains and microfibrils from sugar monomers. This structure published in Science (Purushotham et al 2020) provides a detailed entry point for investigating how the enzyme works, how three of the enzymes assemble into trimeric subunits, and how six of the subunits assemble into the cellulose synthesis complex which makes the cellulose microfibril.
- Our recent manuscript Singh et al. 2020 demonstrates that a full-length plant synthase protein structure can be successfully predicted using computational methods. Our CESA model can be used to explain numerous structure-activity relationships within plant cellulose synthases, and we believe that many researchers will find it useful for the selection and subsequent testing of appropriate mutants in order to optimize biomass properties.
- The nanoscale architecture of fresh, frozen cell walls from several plants has been imaged using low-temperature scanning electron microscopy (cryo-SEM), revealing the dimensions of the cell wall macrofibrils which are made of cellulose and other components. “Visualising the macrofibrils of several trees and of the model plant Arabidopsis allows us to see how the macrofibril size changes in cell walls with different compositions. This will help us develop models for the content of the macrofibrils ” said Professor Paul Dupree, a co-author of the study in the University of Cambridge and a researcher in CLSF. Read the details in Frontiers of Plant Science at Lyczakowski et al. 2019
- Using solid-state NMR spectroscopy, Phyo and CLSF researchers showed that low pH neutralizes pectins, which weaken the interactions between pectins and cellulose, which then allow polysaccharide slippage, ultimately leading to wall loosening. These structural and dynamical findings about Arabidopsis primary cell walls have general implications for the mechanism of acid growth of plant cell walls. Read the details in Cellulose at Phyo et al. 2019
- In a collaborative project, Shrestha and colleagues at Oak Ridge National Lab and Penn State, combined experiment and simulation to determine that arabinsose side-chains confer flexibility to xylan, a property that that may explain xylan function in plant cell walls. Read the details at Shrestha et al. 2019 (Arabinose substitution effect on xylan rigidity and self-aggregation).
- Challenging fundamental cell wall assumptions: an article in the Frontiers in Energy Research Summer 2019 Newsletter entitled Inspiration, Not Imitation: Chemists with Energy Research Centers design molecules for natural function included comments from CLSF's James Kubicki about honing models on cellulose synthesis and the strength of working in a group of scientists with mixed backgrounds and specialties. Since our group started with a good number of scientist that had never worked on the topic of plant cell walls, and continues to add member scientists outside this field, "[we] came in without having the prejudices and biases that people had from reading the literature from the past thirty years... We challenged many of the fundamental assumptions,” Kubicki said, “and one of those was the size of the cellulose microfibril.” Read the full article here
- Our researchers (Hill et al. 2018) revealed that multiple domains in the enzyme that produces cellulose in secondary cell walls, cellulose synthase (CesA), are involved in protein recognition. Chimeric genes were constructed that swapped domains of the enzyme involved in protein-protein contact, and ability to recover crystalline cellulose content and stem height in cellulose deficient plants indicated which domains are relevant in the protein-protein recognition or contact.
- Introduction of a new technique - resonant soft X-ray scattering (RSoXS) - to the study of plant cell walls is reported in a recent paper in Scientific Reports (Ye et al. 2018). RSoXS enhances contrast by tuning the X-ray energy, allowing for the study of cell wall structure on the scale of tens of nanometers. Using this technique, the spacing between microfibrils or microfibril bundles is revealed to be around 20 nm.
- A recent paper in the journal Cellulose (Oehme et al. 2018) details how researchers from the CLSF have developed computational techniques to gain a greater understanding of how cellulose chains pack together within plant cell walls. Molecular dynamics simulations are combined with quantum mechanical calculations of computational models to produce NMR spectra, which can then be compared to experimental data to validate the different models produced.
- In an article on "Diffuse Growth of Plant Cell Walls", Daniel Cosgrove reviews the biophysical basis of cell wall growth, recent paradigm shifts in the role and interactions of cell wall components that compose the cell wall, and insights from atomic force microscopy (AFM) that reveal the details of microfibril organization and motions during wall enlargement.

 Click here to download this slide as a PDF.
Click here to download this slide as a PDF.  Click here to download this slide as a PDF.
Click here to download this slide as a PDF.  Click here to download this slide as a PDF.
Click here to download this slide as a PDF.  Click here to download this slide as a PDF.
Click here to download this slide as a PDF.  Click here to download this slide as a PDF.
Click here to download this slide as a PDF.  Click here to download this slide as a PDF.
Click here to download this slide as a PDF.  Click here to download this slide as a PDF.
Click here to download this slide as a PDF.  Click here to download this slide as a PDF.
Click here to download this slide as a PDF.  Click here to download this slide as a PDF.
Click here to download this slide as a PDF.  Click here to download this slide as a PDF.
Click here to download this slide as a PDF.  Click here to download this slide as a PDF.
Click here to download this slide as a PDF.
 Click here to download this slide as a PDF.
Click here to download this slide as a PDF.
 Click here to download this slide as a PDF.
Click here to download this slide as a PDF.
 Click here to download this slide as a PDF.
Click here to download this slide as a PDF.
 Click here to download this slide as a PDF.
Click here to download this slide as a PDF.
 Click here to download this slide as a PDF.
Click here to download this slide as a PDF.
 Click here to download this slide as a PDF.
Click here to download this slide as a PDF.
More information
- View Research Highlights Poster
- View Research Project Summary
- View a poster with our mission simplified and jargon-free: "Powering your car with sunlight"
 Watch a public lecture by CLSF’s Director Daniel J. Cosgrove entitled “Sunshine Into Wood: How Plants Build Their Cell Walls, a Gigaton Source of Renewable Materials and Energy" presented as part of Penn State University's Sustainability Lecture series. The lecture starts with the big picture of plant cell walls as a source of renewable material and energy, provides a look at our current understanding of how the plant cell wall is constructed, and answers questions on how this energy and material is harnessed in society today, how science progress is aided by big research centers such as the Energy Frontier Research Centers including CLSF. (February 2021)
Watch a public lecture by CLSF’s Director Daniel J. Cosgrove entitled “Sunshine Into Wood: How Plants Build Their Cell Walls, a Gigaton Source of Renewable Materials and Energy" presented as part of Penn State University's Sustainability Lecture series. The lecture starts with the big picture of plant cell walls as a source of renewable material and energy, provides a look at our current understanding of how the plant cell wall is constructed, and answers questions on how this energy and material is harnessed in society today, how science progress is aided by big research centers such as the Energy Frontier Research Centers including CLSF. (February 2021)- Internal wiki page for CLSF members only
Publications
- Yu, Jingyi; Del Mundo, Joshua T.; Freychet, Guillaume; Zhernenkov, Mikhail; Schaible, Eric; Gomez, Esther W.; Gomez, Enrique D.; Cosgrove, Daniel J. (2024) Dynamic Structural Change of Plant Epidermal Cell Walls under Strain. Small 2024, 2311832. doi 10.1002/smll.202311832
- Tryfona, Theodora; Pankratova, Yanina; Petrik, Deborah; Moran, Diego Rebaque; Wightman, Raymond; Yu, Xiaolan; Echevarria-Poza, Alberto; Deralia, Parveen Kumar; Vilaplana, Francisco; Anderson, Charles T.; Hong, Mei; Dupree, Paul (2024) Altering the substitution and cross‐linking of glucuronoarabinoxylans affects cell wall architecture in Brachypodium distachyon. New Phytologist 242(2):524-543. doi 10.1111/nph.19624
- Ajayi, Oyeyemi; Zelinsky, Ellen; Anderson, Charles T. (2024) A core of cell wall proteins functions in wall integrity responses in Arabidopsis thaliana. Plant Direct. 2024 Apr;8(4):e579. doi: 10.1002/pld3.579
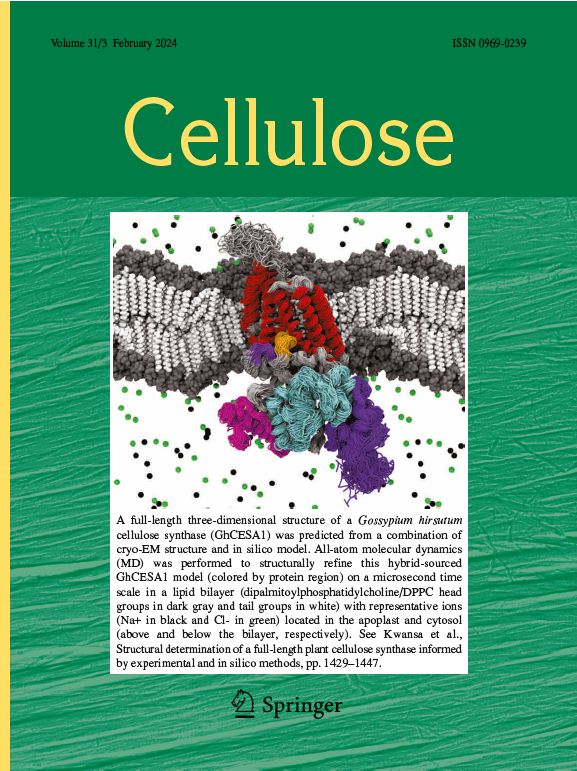
- Kwansa AL, Singh A, Williams JT, Haigler CH, Roberts AW, Yingling YG (2024) Structural determination of a full-length plant cellulose synthase informed by experimental and in silico method. Cellulose 31: 1429-1447. doi 10.1007/s10570-023-05691-x
- Rongpipi, Sintu; William J. Barnes, Oskar Siemianowski, Dan Ye, Joshua T. Del Mundo, Sydney Duncombe, Xiaoran Xin, Chenhui Zhu, Michael F. Toney, Ying Gu, Charles T. Anderson, Enrique D. Gomez, Esther W. Gomez (2024) Matrix polysaccharides affect preferred orientation of cellulose crystals in primary cell walls. Cellulose 31: 1397-1415. doi 10.1007/s10570-023-05702-x
- Yu, Jingyi; Zhang, Yao; and Cosgrove, Daniel J. (2024). The nonlinear mechanics of highly extensible plant epidermal cell walls. Proceedings of the National Academy of Sciences 121, e2316396121. doi 10.1073/pnas.2316396121
- Duan, Pu; Hong, Mei (2024) Selective Detection of Intermediate-Amplitude Motion by Solid-State NMR. J. Phys. Chem. B 128: 2293-2303 doi 10.1021/acs.jpcb.3c06839
- Siemianowski, Oskar; Sintu Rongpipi, Joshua T. Del Mundo, Guillaume Freychet, Mikhail Zhernenkov, Enrique D. Gomez, Esther W. Gomez, Charles T. Anderson (2024) Flexible pectin nanopatterning drives cell wall organization in plants. JACS Au 4 (1): 177-188. doi 10.1021/jacsau.3c00616
- Cosgrove, D.J. (2024) Structure and growth of plant cell walls. Nat Rev Mol Cell Bio doi 10.1038/s41580-023-00691-y
- Choi, Juseok; Lee, Jongcheol; Makarem, Mohamadamin; Lee, Chonghan; Lee, Jongcheol; Kiemle, Sarah; Cosgrove, Daniel J; Kim, Seong H. (2023) Tissue-specific directionality of cellulose synthase complex movement inferred from cellulose microfibril polarity in secondary cell walls of Arabidopsis. Scientific Reports 13, Article number: 22007. doi 10.1038/s41598-023-48545-z
- Gupta, Madhulika; Dupree, Paul; Petridis, Loukas; Smith, Jeremy C. (2023) Patterns in interactions of variably acetylated xylans with hydrophobic cellulose surfaces. Cellulose 30, 11323–11340 (2023). doi 10.1007/s10570-023-05584-z
- Lee J, Choi J, Feng L, Yu J, Zheng Y, Zhang Q, Lin YT, Sah S, Gu Y, Zhang S, Cosgrove DJ, Seong, SH (2023) Regiospecific Cellulose Orientation and Anisotropic Mechanical Property in Plant Cell Walls. Biomacromolecules 24, 4759-4770. doi 10.1021/acs.biomac.3c00538
- Rongpipi S, Barnes WJ, Siemianowski O, Del Mundo JT, Wang C, Freychet G, Zhernenkov M, Anderson CT, Gomez EW, Gomez ED. (2023) Measuring calcium content in plants using NEXAFS spectroscopy. Frontiers in Plant Science. 2023 Aug 16; 14:1212126. doi: 10.3389/fpls.2023.1212126
- Xiang M, Yuan S, Zhang Q, Liu X, Li Q, Leng Z, Sha J, Anderson CT, and Xiao C. (2023). Galactosylation of xyloglucan is essential for the stabilization of the actin cytoskeleton and endomembrane system through the proper assembly of cell walls. J Exp Bot 74, 5104-5123. doi 10.1093/jxb/erad237
- Lee, Jongcheol, Arielle M. Chaves, Juseok Choi, Alison W. Roberts, and Seong H. Kim. (2023) Sum frequency generation (SFG) microscopy analysis of cellulose microfibrils in Physcomitrium patens gametophore leaf. Cellulose Jul 7: 1-10. doi 10.1007/s10570-023-05355-w

- Xin, Xiaoran; Wei, Donghui; Lei, Lei; Zheng, Haiyan; Wallace, Ian; Li, Shundai; Gu, Ying (2023) CALCIUM‐DEPENDENT PROTEIN KINASE32 regulates cellulose biosynthesis through post‐translational modification of cellulose synthase. New Phytologist 239, 2212-2224. doi 10.1111/nph.19106
- Verma, Preeti; Kwansa, Albert L.; Ho, Ruoya; Yingling, Yaroslava G.; Zimmer, Jochen (2023) Insights into substrate coordination and glycosyl transfer of poplar cellulose synthase-8. Structure 31, 1166–1173. doi 10.1016/j.str.2023.07.010
- Chae, Inseok; Paniagua-Guerra, Luis E.; Pitcher, Mica L.; Koshani, Roya; Yuan, Mengxue; Lin, Yen-Ting; Lee, Jongcheol; Perini, Steven E.; Sheikhi, Amir; Ramos-Alvarado, Bladimir; Lanagan, Michael T.; Kim, Seong H. (2023) Relaxation dynamics of water in the vicinity of cellulose nanocrystals. Cellulose 30, 8051-8061. doi 10.1007/s10570-023-05361-y
- Peng, Xiaopeng; Tong, Botong; Lee, Jongcheol; Wang, Kun; Yu, Xiaojuan; Huang, Xiong; Wen, Jialong; Makarem, Mohamadamin; Pang, Hongying; Hinjan, Subin; Yan, Xiaojing; Yao, Shuangquan; Lu, Fachuang; Wang, Baichen; Peng, Feng; Ralph, John; Kim, Seong H.; Sederoff, Ronald R.; Li, Quanzi (2023) Overexpression of a gibberellin 20-oxidase gene in poplar xylem led to an increase in the size of nanocellulose fibrils and improved paper properties. Carbohydrate Polymers 314: 120959. doi 10.1016/j.carbpol.2023.120959
- Wu, Shu-Zon; Chaves, Arielle M.; Li, Rongrong; Roberts, Alison W.; Bezanilla, Magdalena (2023) Cellulose synthase-like D movement in the plasma membrane requires enzymatic activity. Journal of Cell Biology 222 (6): e202212117. doi 10.1083/jcb.202212117
- Del Mundo, Joshua T.; Rongpipi, Sintu; Yang, Hui; Ye, Dan; Kiemle, Sarah N.; Moffitt, Stephanie L.; Troxel, Charles L.; Toney, Michael F.; Zhu, Chenhui; Kubicki, James D.; Cosgrove, Daniel J.; Gomez, Esther W.; Gomez, Enrique D. (2023). Grazing-incidence diffraction reveals cellulose and pectin organization in hydrated plant primary cell wall. Scientific Reports, 13(1), 5421. doi 10.1038/s41598-023-32505-8
- Gilcher, E.; Kuch, N.; Del Mundo, J.T.; Ausman, S.; Santiago-Martinez, L.; Clewett, C.; Gomez, E.W.; Gomez, E.D.; Root, T.; Fox, B.; Dumesic, J. (2023) Evolution of the cellulose microfibril through gamma-valerolactone assisted co-solvent and enzymatic hydrolysis. ACS Sustainable Chemistry & Engineering 11 (8): 3270-3283. doi.org/10.1021/acssuschemeng.2c06030
- Coen, Enrico; Cosgrove, Daniel J. (2023) The mechanics of plant morphogenesis. Science 379, eade8055. doi 10.1126/science.ade8055
- Tryfona, Theodora; Bourdon, Matthieu; Marques, Rita Delgado; Busse‐Wicher, Marta; Vilaplana, Francisco; Stott, Katherine; Dupree, Paul (2023) Grass xylan structural variation suggests functional specialisation and distinctive interaction with cellulose and lignin. The Plant Journal 113: 1004-1020. 10.1016/j.tcsw.2021.100069
- Purushotham, Pallinti; Ho, Ruoya; Yu, Long; Fincher, Geoffrey B.; Bulone, Vincent; Zimmer, Jochen (2022) Mechanism of mixed-linkage glucan biosynthesis by barley cellulose synthase–like CslF6 (1,3;1,4)-β-glucan synthase Science Advances 8(45): eadd1596. doi 10.1126/sciadv.add1596
- Pfaff, Sarah A.; Wang, Xuan; Wagner, Edward R.; Wilson, Liza A.; Kiemle, Sarah N.; Cosgrove, Daniel J. (2022) Detecting the orientation of newly-deposited crystalline cellulose with fluorescent CBM3. The Cell Surface 8: 100089. doi 10.1016/j.tcsw.2022.100089
- Del Mundo, Joshua T.; Rongpipi, Sintu; Gomez, Enrique D.; Gomez, Esther W. (2022) Characterization of biological materials with soft X-ray scattering. In Small Angle Scattering Part A: Methods for Structural Investigation (Methods in Enzymology), 2022, (ed. J. Tainer), Elsevier. doi 10.1016/bs.mie.2022.08.042
- Rongpipi, Sintu; Del Mundo, Joshua T.; Gomez, Enrique D.; Gomez, Esther W. (2022) Extracting structural insights from soft X-ray scattering of biological assemblies. In Small Angle Scattering Part B: Methods for Structural Interpretation (Methods in Enzymology), 2022, (ed. J. Tainer), Elsevier. doi 10.1016/bs.mie.2022.09.017
- Zhao, Wencheng; Deligey, Fabien; Shekar, Chandra; Mentink-Vigier, Frederic; Wang, Tuo (2022) Current limitations of solid-state NMR in carbohydrate and cell wall research. Journal of Magnetic Resonance 341: 107263. doi 10.1016/j.jmr.2022.107263
- Choi, Juseok; Lee, Jongcheol; Makarem, Mohamadamin; Huang, Shixin; Kim, Seong H. (2022) Numerical Simulation of Vibrational Sum Frequency Generation Intensity for Non-Centrosymmetric Domains Interspersed in an Amorphous Matrix: A Case Study for Cellulose in Plant Cell Wall. J. Phys. Chem. B 2022, 126, 35, 6629–6641. doi 10.1021/acs.jpcb.2c03897
- Du, Juan; Vandavasi, Venu Gopal; Molloy, Kelly R.; Yang, Hui; Massenburg, Lynnicia N.; Singh, Abhishek; Kwansa, Albert L.; Yingling, Yaroslava G.; O’Neill, Hugh; Chait, Brian T.; Kumar, Manish; Nixon, Tracy (2022) Evidence for Plant-Conserved Region Mediated Trimeric CESAs in Plant Cellulose Synthase Complexes. Biomacromolecules 23(9): 3663-3677. doi 10.1021/acs.biomac.2c00550
- Temple, Henry; Phyo, Pyae; Yang, Weibing; Lyczakowski, Jan J.; Echevarría-Poza, Alberto; Yakunin, Igor; Parra-Rojas, Juan Pablo; Terrett, Oliver M.; Saez-Aguayo, Susana; Dupree, Ray; Orellana, Ariel; Hong, Mei; Dupree, Paul (2022) Golgi-localized putative S-adenosyl methionine transporters required for plant cell wall polysaccharide methylation. Nature Plants 8: 656-669. doi 10.1038/s41477-022-01156-4
- An associated research briefing highlights the Temple et al. 2022 article: "Shutting the door on polysaccharide methylation". “Identifying potential transporters of SAM into the Golgi is important for understanding the role of pectin modifications in cell wall structure and function”.
- Cosgrove, Daniel J. (2022) Building an Extensible Cell Wall. Plant Physiology, kiac184. doi 10.1093/plphys/kiac184
 Du, Juan; Anderson, Charles T.; Xiao, Chaowen (2022) Dynamics of pectic homogalacturonan in cellular morphogenesis and adhesion, wall integrity sensing and plant development. Nature Plants 8: 332–340 doi 10.1038/s41477-022-01120-2
Du, Juan; Anderson, Charles T.; Xiao, Chaowen (2022) Dynamics of pectic homogalacturonan in cellular morphogenesis and adhesion, wall integrity sensing and plant development. Nature Plants 8: 332–340 doi 10.1038/s41477-022-01120-2- Deligey, Fabien; Frank, Mark A.; Cho, Sung Hyun; Kirui, Alex; Mentink-Vigier, Frederic; Swulius, Matthew T.; Nixon, Tracy; Wang, Tuo (2022) Structure of In Vitro-Synthesized Cellulose Fibrils Viewed by Cryo-Electron Tomography and 13C Natural-Abundance Dynamic Nuclear Polarization Solid-State NMR , Biomacromolecules 23(6): 2290-2301. doi 10.1021/acs.biomac.1c01674 This article was also highlighted on one of the covers of the June 13, 2022 issue of Biomacromolecules.
- Neuwald, Andrew F.; Yang, Hui; Nixon, Tracy (2022) SPARC: Structural properties associated with residue constraints. Computational and Structural Biotechnology Journal 20: 1702-1715. doi 10.1016/j.csbj.2022.04.005
- Li, Xingxing; Chaves, Arielle M; Dees, Dianka C.T; Mansoori, Nasim; Yuan, Kai; Speicher, Tori L; Norris, Joanna H; Wallace, Ian S; Trindade, Luisa M; Roberts, Alison W. (2022) Cellulose synthesis complexes are homo-oligomeric and hetero-oligomeric in Physcomitrium patens. Plant Physiology 188: 2115–2130. doi 10.1093/plphys/kiac003
- Kirui, Alex; Zhao, Wancheng; Deligey, Fabien; Yang, Hui; Kang, Xue; Mentink-Vigier, Frederic; Wang, Tuo (2022) Carbohydrate-aromatic interface and molecular architecture of lignocellulose. Nature Communications 13: 538. doi 10.1038/s41467-022-28165-3
- Chakraborty, Ishita; Rongpipi, Sintu; Govindaraju, Indira; B, Rakesh; Mal, Sib Sankar; Gomez, Esther W.; Gomez, Enrique D.; Kalita, Ranjan Dutta; Nath, Yuthika; Mazumder, Nirmal (2022) An insight into microscopy and analytical techniques for morphological, structural, chemical, and thermal characterization of cellulose. Microscopy Research and Technique, 1-26. doi 10.1002/jemt.24057
- Cosgrove, Daniel J (2022) Plant Cell Growth and Cell Wall Enlargement. eLS, 1-14. doi 10.1002/9780470015902.a0029421
- Cheung, Alice Y.; Cosgrove, Daniel J.; Hara-Nishimura, Ikuko; Jürgens, Gerd; Lloyd, Clive; Robinson, David G.; Staehelin, Andrew; Weijers, Dolf (2022) A rich and bountiful harvest: Key discoveries in plant cell biology. The Plant Cell 34: 53-71. doi 10.1093/plcell/koab234
 Ghassemi, Nader; Poulhazan, Alexandre; Deligey, Fabien; Mentink-Vigier, Frederic; Marcotte, Isabelle; Wang, Tuo (2022) Solid-State NMR Investigations of Extracellular Matrixes and Cell Walls of Algae, Bacteria, Fungi, and Plants. Chemical Reviews 122: 10036-10086. doi 10.1021/acs.chemrev.1c00669 This article was featured on the cover of the May 25, 2022 issue of Chemical Reviews (image created by Daniel S. Rouhani
Ghassemi, Nader; Poulhazan, Alexandre; Deligey, Fabien; Mentink-Vigier, Frederic; Marcotte, Isabelle; Wang, Tuo (2022) Solid-State NMR Investigations of Extracellular Matrixes and Cell Walls of Algae, Bacteria, Fungi, and Plants. Chemical Reviews 122: 10036-10086. doi 10.1021/acs.chemrev.1c00669 This article was featured on the cover of the May 25, 2022 issue of Chemical Reviews (image created by Daniel S. Rouhani- Duncombe, Sydney G; Chethan, Samir G; Anderson, Charles T. (2022) Super-resolution imaging illuminates new dynamic behaviors of cellulose synthase. The Plant Cell 34: 273 – 286. doi 10.1093/plcell/koab227
- Lin, Wenwei; Tang, Wenxin; Pan, Xue; Huang, Aobo; Gao, Xiuqin; Anderson, Charles T; Yang, Zhenbiao (2021) Arabidopsis pavement cell morphogenesis requires FERONIA binding to pectin for activation of ROP GTPase signaling. Current Biology 32(3): 497-507. doi 10.1016/j.cub.2021.11.030
- Xue, Jan; Purushotham, Pallinti; Acheson, Justin F; Ho, Ruoya; Zimmer, Jochen; McFarlane, Ciaran; Van Petegem, Filip; Martone, Patrick T; Samuels, Lacey (2021) Functional characterization of a cellulose synthase, CtCESA1, from the marine red alga Calliarthron tuberculosum (Corallinales). Journal of Experimental Botany 73 (3): 680-695. doi 10.1093/jxb/erab414
- Behar, Hila; Tamura, Kazune; Wagner, Edward R.; Cosgrove, Daniel J.; Brumer, Harry (2021) Conservation of endo-glucanase 16 (EG16) activity across highly divergent plant lineages. Biochemical Journal 478: 3063-3078. doi 10.1042/BCJ20210341
- Julien, Jeffrey A; Fernandez, Martin G; Brandmier, Katrina M; Del Mundo, Joshua T; Bator, Carol M; Loftus, Lucie A; Gomez, Esther W; Gomez, Enrique D; Glover, Kerney Jebrell (2021) Rapid preparation of nanodiscs for biophysical studies. Archives of Biochemistry and Biophysics 712: 109051. doi 10.1016/j.abb.2021.109051
- Burris, Jason N; Makarem, Mohamadamin; Slabaugh, Erin; Chaves, Arielle; Pierce, Ethan T; Lee, Jongcheol; Kiemle, Sarah N; Kwansa, Albert L; Singh, Abhishek; Yingling, Yaroslava G; Roberts, Alison W; Kim, Seong H; Haigler, Candace H (2021) Phenotypic effects of changes in the FTVTxK region of an Arabidopsis secondary wall cellulose synthase compared with results from analogous mutations in other isoforms. Plant Direct 5:e335. doi 10.1002/pld3.335
- Kirui, Alex; Du, Juan; Zhao, Wancheng; Barnes, William; Kang, Xue; Anderson, Charles T; Xiao, Chaowen; Wang, Tuo (2021) A pectin methyltransferase modulates polysaccharide dynamics and interactions in Arabidopsis primary cell walls: Evidence from solid-state NMR. Carbohydrate Polymers 270: 118370. doi: 10.1016/j.carbpol.2021.118370
- Duan, Pu; Kaser, Samuel J; Lyczakowski, Jan J; Phyo, Pyae; Tryfona, Theodora; Dupree, Paul; Hong, Mei (2021) Xylan Structure and Dynamics in Native Brachypodium Grass Cell Walls Investigated by Solid-State NMR Spectroscopy. ACS Omega 6 (23): 15460–15471. doi 10.1021/acsomega.1c01978
- Zhang, Yao; Yu, Jingyi; Wang, Xuan; Durachko, Daniel M; Zhang, Sulin; Cosgrove, Daniel J (2021) Molecular insights into the complex mechanics of plant epidermal cell walls. Science 372 (6543): 706-711. doi 10.1126/science.abf2824
- Rongpipi, Sintu; Del Mundo, Joshua T; Gomez, Enrique D; Gomez, Esther W (2021) Resonant X-ray scattering of biological assemblies. MRS Communications. doi 10.1557/s43579-021-00020-4
- Acheson, J.F; Ho, Ruoya; Goularte, N.F; Cegelski, L; Zimmer, Jochen (2021) Molecular organization of the E. coli cellulose synthase macrocomplex. Nature Structural & Molecular Biology 28: 310 - 318. doi 10.1038/s41594-021-00569-7
- Wilson, Liza A; Deligey, Fabien; Wang, Tuo; Cosgrove, Daniel J (2021) Saccharide Analysis of Onion Outer Epidermal Walls. Biotechnology for Biofuels 14: 66. doi 10.1186/s13068-021-01923-z
- Gupta, Madhulika; Rawal, Takat B; Dupree, Paul; Smith, Jeremy C; Petridis, Loukas (2021) Spontaneous rearrangement of acetylated xylan on hydrophilic cellulose surfaces. Cellulose 28: 3327-3345. doi 10.1007/s10570-021-03706-z
- Allen, Holly; Wei, Donghui; Gu, Ying; Li, Shundai (2021) A historical perspective on the regulation of cellulose biosynthesis. Carbohydrate Polymers 252: 117022. doi 10.1016/j.carbpol.2020.117022
- Zhao, Wancheng; Kirui, Alex; Deligey, Fabien; Mentink-Vigier, Frederic; Zhou, Yihua; Zhang, Baocai; Wang, Tuo (2021) Solid-state NMR of unlabeled plant cell walls: high-resolution structural analysis without isotopic enrichment. Biotechnology for Biofuels 14: 14. doi 10.1186/s13068-020-01858-x
- Addison, Bennett; Stengel, Dillan; Bharadwaj, Vivek S; Happs, Renee M; Doeppke, Crissa; Wang, Tuo; Bomble, Yannick J; Holland, Gregory P; Harman-Ware, Anne E. (2020) Selective One-Dimensional 13C−13C Spin-Diffusion Solid-State Nuclear Magnetic Resonance Methods to Probe Spatial Arrangements in Biopolymers Including Plant Cell Walls, Peptides, and Spider Silk. The Journal of Physical Chemistry B 124: 9870 – 9883. doi 10.1021/acs.jpcb.0c07759
- Zhu, Xiaoyu; Tellier, Frédérique; Gu, Ying; Li, Shundai (2020) Disruption of Very-Long-Chain-Fatty Acid Synthesis Has an Impact on the Dynamics of Cellulose Synthase in Arabidopsis thaliana. Plants 9: 1599. doi 10.3390/plants9111599
- Ye, Dan; Rongpipi, Sintu; Kiemle, Sarah N; Barnes, William J; Chaves, Arielle M; Zhu, Chenhui; Norman, Victoria A; Liebman-Peláez, Alexander; Hexemer, Alexander; Toney, Michael F; Roberts, Alison W; Anderson, Charles T; Cosgrove, Daniel J; Gomez, Esther W; Gomez, Enrique D. (2020) Preferred crystallographic orientation of cellulose in plant primary cell walls. Nature Communications 11: 4720. 10.1038/s41467-020-18449-x
- Du, Juan; Kirui, Alex; Huang, Shixin; Wang, Lianglei; Barnes, William J.; Kiemle, Sarah; Zheng, Yunzhen; Rui, Yue; Ruan, Mei; Qi, Shiqian; Kim, Seong H.; Wang, Tuo; Cosgrove, Daniel J.; Anderson, Charles T.; Xiao, Chaowen (2020) Mutations in the Pectin Methyltransferase QUASIMODO2 Influence Cellulose Biosynthesis and Wall Integrity in Arabidopsis thaliana. The Plant Cell 32: 3576–3597. doi 10.1105/tpc.20.00252
- Fernando, Liyanage D; Zhao, Wancheng; Widanage, Malitha C. Dickwel; Mentink-Vigier, Frédéric; Wang, Tuo (2020) Solid-state NMR and DNP Investigations of Carbohydrates and Cell-wall Biomaterials. eMagRes 9: 251 -258. doi 10.1002/9780470034590.emrstm1624
- Makarem, Mohamadamin; Nishiyama, Yoshiharu; Xin, Xiaoran; Durachko, Daniel M.; Gu, Ying; Cosgrove, Daniel J.; Kim, Seong H. (2020) Distinguishing Mesoscale Polar Order (Unidirectional vs Bidirectional) of Cellulose Microfibrils in Plant Cell Walls Using Sum Frequency Generation Spectroscopy. The Journal of Physical Chemistry B 124: 8071–8081. doi 10.1021/acs.jpcb.0c07076

- Purushotham, Pallinti; Ho, Ruoya; Zimmer, Jochen (2020) Architecture of a catalytically active homotrimeric plant cellulose synthase complex. Science 369(6507): 1089-1094. doi 10.1126/science.abb2978
- Cosgrove, Daniel J (2020) Theory and Practice in Measuring In-Vitro Extensibility of Growing Plant Cell Walls. In: Popper, Zoë A. (eds) The Plant Cell Wall. Methods in Molecular Biology 2149: 57 – 72. DOI 10.1007/978-1-0716-0621-6_4
- Roberts, Alison W; Dimos, Christos S; Budziszek, Michael J; Goss, Chessa A; Lai, Virginia; Chaves, Arielle M (2020) Knocking Out the Wall: Revised Protocols for Gene Targeting in Physcomitrella patens. In: Popper, Zoë A. (eds) The Plant Cell Wall. Methods in Molecular Biology 2149: 125 – 144. DOI 10.1007/978-1-0716-0621-6_8
- Duncombe, Sydney G; Barnes, William J; Anderson, Charles T. (2020) Imaging the delivery and behavior of cellulose synthases in Arabidopsis thaliana using confocal microscopy. Methods Cell Biology 160:201-213. doi 10.1016/bs.mcb.2020.04.005.
- Zheng, Yunzhen; Ning, Gang; Cosgrove, Daniel J (2020) High-Resolution Imaging of Cellulose Organization in Cell Walls by Field Emission Scanning Electron Microscopy. In: Popper, Zoë A. (eds) The Plant Cell Wall. Methods in Molecular Biology 2149: 225 – 237. DOI 10.1007/978-1-0716-0621-6_13
- Singh, Abhishek; Kwansa, Albert L; Kim, Ho Shin; Williams, Justin T; Yang, Hui; Li, Nan K; Kubicki, James D; Roberts, Alison W; Haigler, Candace H; Yingling, Yaroslava G (2020) In silico prediction of full-length integral membrane protein structure, cotton cellulose synthase (GhCESA1), and its hierarchical complexes. Cellulose 27: 5597–5616. doi.org/10.1007/s10570-020-03194-7
- Petrik, Deborah L; Tryfona, Theodora; Dupree, Paul; Anderson, Charles T (2020) BdGT43B2 functions in xylan biosynthesis and is essential for seedling survival in Brachypodium distachyon. Plant Direct 4(4): e00216. doi.org/10.1002/pld3.216
- Cosgrove, Daniel J; Anderson, Charles T (2020) Plant Cell Growth: Do Pectins Drive Lobe Formation in Arabidopsis Pavement Cells? Current Biology 30(11):R660-R662. doi 10.1016/j.cub.2020.04.007.
- Chakraborty, Arnab; Deligey, Fabien; Quach, Jenny; Mentink-Vigier, Frederic; Wang, Ping; Wang, Tuo (2020) Biomolecular complex viewed by dynamic nuclear polarization solid-state NMR spectroscopy. Biochemical Society Transactions online. doi.org/10.1042/BST20191084
- Zhao, Wancheng; Fernando, Liyanage D; Kirui, Alex; Deligey, Fabien; Wang, Tuo (2020) Solid-state NMR of plant and fungal cell walls: A critical review. Solid State Nuclear Magnetic Resonance 107: 101660. doi.org/10.1016/j.ssnmr.2020.101660
- Xiong, Rui; Singh, Abhishek; Yu, Shengtao; Zhang, Shuaidi; Lee, Hansol; Yingling, Yaroslava G.; Nepal, Dhriti; Bunning, Timothy J.; Tsukruk, Vladimir V. (2020) Co-assembling Polysaccharide Nanocrystals and Nanofibers for Robust Chiral Iridescent Films. ACS Applied Materials & Interfaces 12: 35345 – 35353. doi 10.1021/acsami.0c08571
- Basu, Snehasish; Catchmark, Jeffrey M; Brown, Nicole R; Anderson, Charles T; Gorniak, Ireneusz P (2020) BcsAB synthesized cellulose on nickel surface: Polymerization of monolignols during cellulose synthesis alters cellulose morphology. Cellulose 27: 5629–5639. doi.org/10.1007/s10570-020-03178-7
- Mani, Sriramvignesh; Cosgrove, Daniel J; Voth, Gregory A (2020) Anisotropic Motions of Fibrils Dictated by Their Orientations in the Lamella: A Coarse-Grained Model of a Plant Cell Wall. The Journal of Physical Chemistry B 124(17): 3527–3539. doi.org/10.1021/acs.jpcb.0c01697
- Rawal, Takat B; Zahran, Mai; Dhital, Brittiny; Akbilgic, Oguz; Petridis, Loukas (2020) The relation between lignin sequence and its 3D structure. Biochimica et Biophysica Acta (BBA) - General Subjects 1864(5): 129547. doi.org/10.1016/j.bbagen.2020.129547
- Anderson, Charles T; Kieber, Joseph J (2020) Dynamic Construction, Perception, and Remodeling of Plant Cell Walls. Annual Review of Plant Biology 71: 39-69. doi.org/10.1146/annurev-arplant-081519-035846
 Chae, Inseok; Ngo, Dien; Chen, Zhe; Kwansa, Albert L; Chen, Xing; Meddeb, Amira Barhoumi; Podraza, Nikolas J; Yingling, Yaroslava G; Ounaies, Zoubeida; Kim, Seong H (2020) Anisotropic Optical and Frictional Properties of Langmuir–Blodgett Film Consisting of Uniaxially‐Aligned Rod‐Shaped Cellulose Nanocrystals. Advanced Materials Interfaces, 1902169. doi.org/10.1002/admi.201902169
Chae, Inseok; Ngo, Dien; Chen, Zhe; Kwansa, Albert L; Chen, Xing; Meddeb, Amira Barhoumi; Podraza, Nikolas J; Yingling, Yaroslava G; Ounaies, Zoubeida; Kim, Seong H (2020) Anisotropic Optical and Frictional Properties of Langmuir–Blodgett Film Consisting of Uniaxially‐Aligned Rod‐Shaped Cellulose Nanocrystals. Advanced Materials Interfaces, 1902169. doi.org/10.1002/admi.201902169- Yang, Hui; Kubicki, James D (2020) A density functional theory study on the shape of the primary cellulose microfibril in plants: effects of C6 exocyclic group conformation and H-bonding. Cellulose 27: 2389-2402. doi.org/10.1007/s10570-020-02970-9
- Xin, Xiaoran; Lei, Lei; Zheng, Yunzhen; Zhang, Tian; Pingali, Sai Venkatesh; O'Neill, Hugh; Cosgrove, Daniel J; Li, Shundai; Gu, Ying (2020) CELLULOSE SYNTHASE INTERACTIVE1- and Microtubule-Dependent Cell Wall Architecture Is Required for Acid Growth in Arabidopsis Hypocotyls. Journal of Experimental Biology 71(10): 2982–2994. doi.org/10.1093/jxb/eraa063/5722043
- Makarem, Mohamadamin; Kim, Hyojung; Emami, Parinaz; Melendez, Jesus; Steinbach, Adam; Lipkie, Tristan; Deleris, Isabelle; Desmet, Christina; Wallecan, Jöel; Kim, Seong H. (2020) Impact of Drying on Meso- and Nanoscale Structures of Citrus Fiber: A Study by SFG, ATR-IR, XRD, and DLS. Industrial & Engineering Chemistry Research 59 (7): 2718-2724. doi.org/10.1021/acs.iecr.9b06194
- Wang, Xuan; Wilson, Liza; Cosgrove, Daniel J (2020) Pectin methylesterase selectively softens the onion epidermal wall yet reduces acid-induced creep. Journal of Experimental Biology 71(9): 2629-2640. doi.org/10.1093/jxb/eraa059
 Huang, Shixin; Kiemle, Sarah N; Makarem, Mohamadamin; Kim, Seong H. (2020) Correlation between crystalline cellulose structure and cellulose synthase complex shape: a spectroscopic study with unicellular freshwater alga Micrasterias. Cellulose 27: 57-69. doi/org/10.1007/s10570-019-02793-3
Huang, Shixin; Kiemle, Sarah N; Makarem, Mohamadamin; Kim, Seong H. (2020) Correlation between crystalline cellulose structure and cellulose synthase complex shape: a spectroscopic study with unicellular freshwater alga Micrasterias. Cellulose 27: 57-69. doi/org/10.1007/s10570-019-02793-3- Rawal, Takat B; Zahran, Mai; Dhital, Brittiny; Akbilgic, Oguz; Petridis, Loukas (2020) The relation between lignin sequence and its 3D structure. Biochimica et Biophysica Acta (BBA) - General Subjects: 129547. doi.org/10.1016/j.bbagen.2020.129547
- Abbas, Manzar; Peszlen, Ilona; Shi, Rui; Kim, Hoon; Katahira, Rui; Kafle, Kabindra; Xiang, Zhouyang; Huang, Xiong; Min, Douyong; Makarem, Mohamadamin; Yang, Chenmin; Dai, Xinren; Yan, Xiaojing; Park, Sunkyu; Li, Yun; Kim, Seong H; Davis, Mark; Ralph, John; Sederoff, Ronald R; Chiang, Vincent L; Li, Quanzi; Plomion, Christophe (2020) Involvement of CesA4, CesA7-A/B and CesA8-A/B in secondary wall formation in Populus trichocarpa wood. Tree Physiology 40: 73-89. doi.org/10.1093/treephys/tpz020
- Brabham, Chad; Singh, Abhishek; Stork, Jozsef; Rong, Ying; Kumar, Indrajit; Kikuchi, Kazuhiro; Yingling, Yaroslava G; Brutnell, Thomas P; Rose, Jocelyn K.C; DeBolt, Seth (2019) Biochemical and physiological flexibility accompanies reduced cellulose biosynthesis in Brachypodium cesa1S830N. AoB PLANTS 11 (5). doi.org/10.1093/aobpla/plz041
- Zhang, Tian; Tang, Haosu; Vavylonis, Dimitrios; Cosgrove, Daniel J. (2019) Disentangling loosening from softening: insights into primary cell wall structure. The Plant Journal 100: 1101-1117. doi.org/10.1111/tpj.14519
- Phyo, Pyae; Hong, Mei (2019) Fast MAS 1H–13C correlation NMR for structural investigations of plant cell walls. Journal of Biomolecular NMR 73 (12): 661–674. doi.org/10.1007/s10858-019-00277-x
- Kubicki, James D; Yang, Hui; Kim, Seong H (2019) Integrating Density Functional Theory Calculations with Vibrational and Nuclear Magnetic Resonance Spectroscopy. Understanding Lignocellulose: Synergistic Computational and Analytic Methods 1338: 89 – 102 doi.org/10.1021/symposium10.1021/bk-2019-133810.1021/bk-2019-1338.ch006
- Lyczakowski, Jan J; Bourdon, Matthieu; Terrett, Oliver M; Helariutta, Ykä; Wightman, Raymond; Dupree, Paul (2019) Structural Imaging of Native Cryo-Preserved Secondary Cell Walls Reveals the Presence of Macrofibrils and Their Formation Requires Normal Cellulose, Lignin and Xylan Biosynthesis. Frontiers in Plant Science 10: 1398. 10.3389/fpls.2019.0139810.3389/fpls.2019.01398.s001
- Raeeszadeh‐Sarmazdeh, Maryam; Patel, Nikhil; Cruise, Sarah; Owen, Leila; O'Neill, Hugh; Boder, Eric T (2019) Identifying Stable Fragments of Arabidopsis thaliana Cellulose Synthase Subunit 3 by Yeast Display. Biotechnology Journal 14: 1800353. doi.org/10.1002/biot.201800353
- Li, Xingxing; Speicher, Tori L; Dees, Dianka; Mansoori, Nasim; McManus, John B; Tien, Ming; Trindade, Luisa M; Wallace, Ian S; Roberts, Alison W (2019) Convergent evolution of hetero-oligomeric cellulose synthesis complexes in mosses and seed plants. The Plant Journal 99: 862-876. https://doi.org/10.1111/tpj.14366
 Yang, Hui; McManus, John; Oehme, Daniel; Singh, Abhishek; Yingling, Yaroslava G; Tien, Ming; Kubicki, James D (2019) Simulations of Cellulose Synthesis Initiation and Termination in Bacteria. The Journal of Physical Chemistry Part B 123 (17): 3699–3705. https://doi.org/10.1021/acs.jpcb.9b02433 This article also supplied the cover art for one of two printed issues.
Yang, Hui; McManus, John; Oehme, Daniel; Singh, Abhishek; Yingling, Yaroslava G; Tien, Ming; Kubicki, James D (2019) Simulations of Cellulose Synthesis Initiation and Termination in Bacteria. The Journal of Physical Chemistry Part B 123 (17): 3699–3705. https://doi.org/10.1021/acs.jpcb.9b02433 This article also supplied the cover art for one of two printed issues. - Rongpipi, Sintu; Ye, Dan; Gomez, Enrique D; Gomez, Esther W (2019) Progress and Opportunities in the Characterization of Cellulose – An Important Regulator of Cell Wall Growth and Mechanics. Frontiers in Plant Science 9: 1894. doihttps://doi.org/10.3389/fpls.2018.01894
- Raeeszadeh‐Sarmazdeh, Maryam; Patel, Nikhil; Cruise, Sarah; Owen, Leila; O'Neill, Hugh; Boder, Eric T (2019) Identifying Stable Fragments of Arabidopsis thaliana Cellulose Synthase Subunit 3 by Yeast Display. Biotecnology Journal 14: 1800353. https://onlinelibrary.wiley.com/doi/full/10.1002/biot.201800353
- Shrestha, Utsab R; Smith, Sydney; Pingali, Sai Venkatesh; Yang, Hui; Zahran, Mai; Breunig, Lloyd; Wilson, Liza A; Kowalik, Malgorzata; Kubicki, James D; Cosgrove, Daniel J; O’Neill, Hugh M; Petridis, Loukas (2019) Arabinose substitution effect on xylan rigidity and self-aggregation. Cellulose 26(4): 2267-2278. https://doi.org/10.1007/s10570-018-2202-8
- Shah, Riddhi; Huang, Shixin; Pingali, Sai Venkatesh; Sawada, Daisuke; Pu, Yunqiao; Rodriguez, Miguel; Ragauskas, Arthur J; Kim, Seong H; Evans, Barbara R; Davison, Brian H; O’Neill, Hugh (2019) Hemicellulose–Cellulose Composites Reveal Differences in Cellulose Organization after Dilute Acid Pretreatment. Biomacromolecules 20(2): 893-903. https://doi.org/10.1021/acs.biomac.8b01511
- Li, Fu-Shuang; Phyo, Pyae; Jacobowitz, Joseph; Hong, Mei; Weng, Jing-Ke (2019) The molecular structure of plant sporopollenin. Nature Plants 5(1): 41-46. https://doi.org/10.1038/s41477-018-0330-7
- Phyo, Pyae; Gu, Ying; Hong, Mei (2019) Impact of acidic pH on plant cell wall polysaccharide structure and dynamics: insights into the mechanism of acid growth in plants from solid-state NMR. Cellulose 26(1): 291-304. https://doi.org/10.1007/s10570-018-2094-7
- Zimmer, Jochen (2019) Structural features underlying recognition and translocation of extracellular polysaccharides. Interface Focus Vol. 9, Issue 2: 20180060 https://doi.org/10.1098/rsfs.2018.0060
- Ling, Zhe; Wang, Tuo; Makarem, Mohamadamin; Cintrón, Michael Santiago; Cheng, H. N.; Kang, Xue; Bacher, Markus; Potthast, Antje; Rosenau, Thomas ; King, Holly ; Delhom, Christopher D; Nam, Sunghyun ; Edwards, Vincent; Kim, Seong H; Xu, Feng; French, Alfred D (2019) Effects of ball milling on the structure of cotton cellulose. Cellulose 26: 305–328. https://doi.org/10.1007/s10570-018-02230-x
- Kang, Xue; Kirui, Alex; Widanage, Malitha C. Dickwel; Mentink-Vigier, Frederic; Cosgrove, Daniel J; Wang, Tuo (2019) Lignin-polysaccharide interactions in plant secondary cell walls revealed by solid-state NMR. Nature Communications 10:347. https://doi.org/10.1038/s41467-018-08252-0
- Makarem, Mohamadamin; Lee, Christopher M; Kafle, Kabindra; Huang, Shixin; Chae, Inseok; Yang, Hui; Kubicki, James D; Kim, Seong H (2019) Probing cellulose structures with vibrational spectroscopy. Cellulose 26(1): 35-79. https://doi.org/10.1007/s10570-018-2199-z
- Haigler, Candace H; Roberts, Alison W (2019) Structure/function relationships in the rosette cellulose synthesis complex illuminated by an evolutionary perspective. Cellulose 26(1): 227-247. https://doi.org/10.1007/s10570-018-2157-9
- Yang, Hui; Watts, Heath D; Gibilterra, Virgil; Weiss, T. Blake; Petridis, Loukas; Cosgrove, Daniel J; Kubicki; James D (2019) Quantum Calculations on Plant Cell Wall Component Interactions. Interdisciplinary Sciences: Computational Life Sciences 11: 485-495. https://link.springer.com/article/10.1007%2Fs12539-018-0293-4
- McManus, John B.; Wilson, Liza ; Yang, Hui ; Kubicki, James D; Tien, Ming (2018) Kinetic analysis of cellulose synthase of Gluconacetobacter hansenii in whole cells and in purified form. Enzyme and Microbial Technology 119 (2018) 24–29. https://doi.org/10.1016/j.enzmictec.2018.08.005
- Kittle JD, Qian C, Edgar E, Roman M, Esker AR (2018) Adsorption of Xyloglucan onto Thin Films of Cellulose Nanocrystals and Amorphous Cellulose: Film Thickness Effects. ACS Omega 2018, 3:14004-14012. https://doi.org/10.1016/j.cub.2018.07.076
- Hill, Joseph Lee; Hill, Ashley Nicole; Roberts, Alison W; Haigler, Candace H; Tien, Ming (2018) Domain swaps of Arabidopsis secondary wall cellulose synthases to elucidate their class specificity. Plant Direct 2: e00061. https://doi.org/10.1002/pld3.61
- Cosgrove, Daniel J (2018) Nanoscale structure, mechanics and growth of epidermal cell walls. Current Opinion in Plant Biology 46: 77-86. https://doi.org/10.1016/j.pbi.2018.07.016
- Yang, Yang; Yu, Youjian; Liang, Ying; Anderson, Charles T; Cao, Jiashu (2018) A Profusion of Molecular Scissors for Pectins: Classification, Expression, and Functions of Plant Polygalacturonases. Frontiers in Plant Science 9: 1208. https://doi.org/10.3389/fpls.2018.01208
- Watanabe, Yoichiro; Schneider, Rene; Barkwill, Sarah; Gonzales-Vigil, Eliana; Hill, Joseph L; Samuels, Lacey; Persson, Staffan; Mansfield, Shawn D (2018) Cellulose synthase complexes display distinct dynamic behaviors during xylem transdifferentiation. Proceedings of the National Academy of Sciences 115(27): E6366-E6374. https://doi.org/10.1073/pnas.1802113115
- Anderson, Charles T (2018) Finding order in a bustling construction zone: quantitative imaging and analysis of cell wall assembly in plants. Current Opinion in Plant Biology 46: 62-67. https://doi.org/10.1016/j.pbi.2018.07.014
- Cosgrove, Daniel J (2018) Nanoscale structure, mechanics and growth of epidermal cell walls. Current Opinion in Plant Biology 46: 77-86. https://doi.org/10.1016/j.pbi.2018.07.016
- Takenaka, Yuto; Watanabe, Yoichiro; Schuetz, Mathias; Unda, Faride; Hill, Joseph Lee; Phookaew, Pawittra; Yoneda, Arata; Mansfield, Shawn D; Samuels, Lacey; Ohtani, Misato; Demura, Taku (2018) Patterned deposition of xylan and lignin is independent from the secondary wall cellulose of Arabidopsis xylem vessels. The Plant Cell tpc.00292.2018. https://doi.org/10.1105/tpc.18.00292
- Ye, Dan; Kiemle, Sarah N; Rongpipi, Sintu; Wang, Xuan; Wang, Cheng; Cosgrove, Daniel J; Gomez, Esther W; Gomez, Enrique D (2018) Resonant soft X-ray scattering reveals cellulose microfibril spacing in plant primary cell walls. Scientific Reports 8: 12449. https://doi.org/10.1038/s41598-018-31024-1
- Polko, Joanna K; Barnes, William J; Voiniciuc, Cătălin; Doctor, Stephanie; Steinwand, Blaire; Hill, Joseph L; Tien, Ming; Pauly, Markus; Anderson, Charles T; Kieber, Joseph J (2018) SHOU4 Proteins Regulate Trafficking of Cellulose Synthase Complexes to the Plasma Membrane. Current Biology 28(19): 3174 - 3182.e6. https://doi.org/10.1016/j.cub.2018.07.076
- Kubicki, James D; Yang, Hui; Sawada, Daisuke; O’Neill, Hugh; Oehme, Daniel; Cosgrove, Daniel (2018) The Shape of Native Plant Cellulose Microfibrils. Scientific Reports 8: 13983. https://www.nature.com/articles/s41598-018-32211-w
- Haigler, Candace H (2018) Two types of cellulose synthesis complex knit the plant cell wall together. Proceedings of the National Academy of Sciences 115 (27): 6882-6884. http://www.pnas.org/content/115/27/6882
- Oehme, Daniel P; Yang, Hui; Kubicki, James D (2018) An evaluation of the structures of cellulose generated by the CHARMM force field: comparisons to in planta cellulose. Cellulose 25: 3755-3779. https://doi.org/10.1007/s10570-018-1793-4
- Huang, Shixin; Makarem, Mohamadamin; Kiemle, Sarah N; Zheng, Yunzhen; He, Xin; Ye, Dan; Gomez, Esther W; Gomez, Enrique D; Cosgrove, Daniel J; Kim, Seong H (2018) Dehydration-induced physical strains of cellulose microfibrils in plant cell walls. Carbohydrate Polymers 197: 337-348. https://doi.org/10.1016/j.carbpol.2018.05.091
- Phyo, Pyae; Wang, Tuo; Yang, Yu; O’Neill, Hugh; Hong, Mei (2018) Direct Determination of Hydroxymethyl Conformations of Plant Cell Wall Cellulose Using 1H Polarization Transfer Solid-State NMR. Biomacromolecules 19(5): 1485-1497. https://pubs.acs.org/doi/abs/10.1021/acs.biomac.8b00039
- Xiong, Rui; Kim, Ho Shin; Zhang, Lijuan; Korolovych, Volodymyr F; Zhang, Shuaidi; Yingling, Yaroslava; Tsukruk, Vladimir V. (2018) Hairy Graphenes: Wrapping Nanocellulose Nets around Graphene Oxide Sheets. Angewandte Chemie International Edition 57: 8508. https://doi.org/10.1002/ange.201803076
- Zhu, Xiaoyu; Li, Shundai; Pan, Songqin; Xin, Xiaoran; Gu, Ying (2018) CSI1, PATROL1, and exocyst complex cooperate in delivery of cellulose synthase complexes to the plasma membrane. PNAS 115(15) E3578-E3587. https://doi.org/10.1073/pnas.1800182115
- Barnes, William J; Anderson, Charles T (2018) Cytosolic invertases contribute to cellulose biosynthesis and influence carbon partitioning in seedlings of Arabidopsis thaliana. Plant Journal 94(6): 956-974. https://doi.org/10.1111/tpj.13909
- Ganguly, Anindya; DeMott, Logan; Zhu, Chuanmei; McClosky, Daniel D; Anderson, Charles T; Dixin, Ram (2018) Importin-β directly regulate the motor activity and turnover of a kinesin-4. Developmental Cell 2018 Mar 12;44(5):642-651.e5. http://www.cell.com/developmental-cell/fulltext/S1534-5807(18)30093-5
- McManus, John; Yang, Hui; Wilson, Liza; Kubicki, James; Tien, Ming (2018) Initiation, Elongation and Termination of Bacterial Cellulose Synthesis. ACS Omega 3(3): 2690-2698. https://pubs.acs.org/doi/10.1021/acsomega.7b01808
- Hill, Joseph L; Josephs, Cooper; Barnes, William J; Anderson, Charles T; Tien, Ming (2018) Longevity in vivo of Primary Cell Wall Cellulose Synthases. Plant Molecular Biology 96(3): 279-289. https://link.springer.com/article/10.1007%2Fs11103-017-0695-4
- Scavuzzo-Duggan, Tess R; Chaves, Arielle M; Singh, Abhishek; Sethaphong, Latsavongsakda; Slabaugh, Erin; Yingling, Yaroslava G; Haigler, Candace H; Roberts, Alison W (2018) Cellulose synthase ‘class specific regions’ are intrinsically disordered and functionally undifferentiated. Journal of Integrative Plant Biology 60(6):481-497. http://onlinelibrary.wiley.com/doi/10.1111/jipb.12637/abstract/

- Zheng, Yunzhen; Wang, Xuan; Chen, Yuning; Wagner, Edward; Cosgrove, Daniel J (2018) Xyloglucan in the primary cell wall: assessment by FESEM, selective enzyme digestions and nanogold affinity tags. The Plant Journal 93(2): 211 – 226. Featured as cover image of Plant Jounral Volume 93 Issue 2 and subject of a research highlight article. http://onlinelibrary.wiley.com/doi/10.1111/tpj.13778/abstract/
- Barnes, William J; Anderson, Charles T (2018) Release, Recycle, Rebuild: Cell-Wall Remodeling, Autodegradation, and Sugar Salvage for New Wall Biosynthesis during Plant Development. Molecular Plant 11(1): 31-46. https://doi.org/10.1016/j.molp.2017.08.011
- Tran, Mai L; McCarthy, Thomas W; Sun, Hao; Wu, Shu-Zon; Norris, Joanna H; Bezanilla, Magdalena; Vidali, Luis; Anderson, Charles T; Roberts, Alison W (2018) Direct observation of the effects of cellulose synthesis inhibitors using live cell imaging of Cellulose Synthase (CESA) in Physcomitrella patens. Scientific Reports 8(1):735. https://www.nature.com/articles/s41598-017-18994-4
- Cosgrove, Daniel J (2018) Diffuse growth of plant cell walls. Plant Physiology pp.01541.2017. http://www.plantphysiol.org/content/176/1/16
- Makarem, Mohamadamin; Lee, Christopher M; Sawada, Daisuke; O’Neill, Hugh M; Kim, Seong H (2018) Distinguishing Surface versus Bulk Hydroxyl Groups of Cellulose Nanocrystals Using Vibrational Sum Frequency Generation Spectroscopy. The Journal of Physical Chemistry Letters 9(1): 70-75. http://dx.doi.org/10.1021/acs.jpclett.7b02729
- Yang, Hui; Wang, Tuo; Oehme, Daniel; Petridis, Loukas; Hong, Mei; Kubicki, James D (2018) Structural factors affecting 13C NMR chemical shifts of cellulose: a computational study. Cellulose 25(1): 23-36. https://link.springer.com/article/10.1007%2Fs10570-017-1549-6
- Phyo, Pyae; Wang, Tuo; Kiemle, Sarah N; O'Neill, Hugh; Pingali, Sai Venkatesh; Hong, Mei; Cosgrove, Daniel J (2017) Gradients in wall mechanics and polysaccharides along growing inflorescence stems. Plant Physiology 175(4): 1593-1607. http://www.plantphysiol.org/content/175/4/1593
- Durachko, Daniel M; Park, Yong Bum; Zhang, Tian ; Cosgrove, Daniel J (2017) Biomechanical Characterization of Onion Epidermal Cell Walls, BIO-PROTOCOL 7(24): e2662. https://bio-protocol.org/e2662
- Zhang, Tian; Cosgrove, Daniel J (2017) Preparation of Onion Epidermal Cell Walls for Imaging by Atomic Force Microscopy (AFM). BIO-PROTOCOL 7(24): e2647. https://bio-protocol.org/e2647
- O’Neill, Hugh; Pingali, Sai Venkatesh; Petridis, Loukas; He, Junhong; Mamontov, Eugene; Hong, Liang; Urban, Volker; Evans, Barbara; Langan, Paul; Smith, Jeremy C; Davison, Brian H (2017) Dynamics of water bound to crystalline cellulose. Scientific Reports 7: 11840. https://www.nature.com/articles/s41598-017-12035-w
- Rui, Yue; Xiao, Chaowen; Yi, Hojae; Kandemir, Baris; Wang, James Z; Puri, Virendra M; Anderson, Charles T (2017) POLYGALACTURONASE INVOLVED IN EXPANSION3 Functions in Seedling Development, Rosette Growth, and Stomatal Dynamics in Arabidopsis thaliana. The Plant Cell 29: 2413-2432. http://dx.doi.org/10.1105/tpc.17.00568
- Phyo, Pyae; Wang, Tuo; Xiao, Chaowen; Anderson, Charles T; Hong, Mei (2017) Effects of Pectin Molecular Weight Changes on the Structure, Dynamics, and Polysaccharide Interactions of Primary Cell Walls of Arabidopsis thaliana: Insights from Solid-State NMR. Biomacromolecules 18(9): 2937-2950. http://dx.doi.org/10.1021/acs.biomac.7b00888
- Norris, Joanna H; Li, Xingxing; Huang, Shixin; Van de Meene, Allison ML; Tran, Mai L; Killeavy, Erin; Chaves, Arielle M; Mallon, Bailey; Mercure, Danielle; Tan, Hwei-Ting; Burton, Rachel A; Doblin, Monika S; Kim, Seong H; Roberts, Alison W (2017) Functional specialization of cellulose synthase isoforms in a moss shows parallels with seed plants. Plant Physiology 175: 210-222. https://doi.org/10.1104/pp.17.00885
- Cho, Sung Hyun; Purushotham, Pallinti;Fang, Chao; Maranas, Cassandra; Díaz-Moreno, Sara M; Bulone, Vincent; Zimmer, Jochen; Kumar, Manish; Nixon, B. Tracy (2017) Synthesis and Self-Assembly of Cellulose Microfibrils from Reconstituted Cellulose Synthase. Plant Physiology 175: 146-156. http://dx.doi.org/10.1104/pp.17.00619
- Zheng, YunZhen; Cosgrove, Daniel J; Ning, Gang (2017) High-Resolution Field Emission Scanning Electron Microscopy (FESEM) Imaging of Cellulose Microfibril Organization in Plant Primary Cell Walls. Microscopy and Microanalysis: 1-7. https://doi.org/10.1017/S143192761701251X
- Cosgrove, Daniel J (2017) Microbial Expansins. Annu Rev Microbiol 71: 479-497 http://dx.doi.org/10.1146/annurev-micro-090816-093315
- Chen, Xing; Lee, Christopher M; Wang, Hong-Fei; Lasse, Jensen; Kim, Seong H (2017) Vibrational Sum-Frequency-Generation (SFG) Spectroscopy of Bulk Materials Consisting of SFG active Nanocrystals: Experimental and Theoretical Study of Azimuth Angle and Polarization Dependences of SFG Spectral Features of Uniaxially-Aligned Cellulose Crystals. The Journal of Physical Chemistry Part C 121(34): 18876-18886. http://dx.doi.org/10.1021/acs.jpcc.7b03037
- Kafle, Kabindra; Yong Bum, Park; Lee, Christoper M; Stapleton, Joshua J; Kiemle, Sarah N; Cosgrove, Daniel J; Kim, Seong H (2017) Effects of mechanical stretching on average orientation of cellulose and pectin in onion epidermis cell wall: A polarized FT-IR study. Cellulose 24: 3145-3154. http://dx.doi.org/10.1007/s10570-017-1337-3
- Goodell; Barry, Zhu, Yuan; Kim, Seong H; Kafle, Kabindra; Eastwood, Daniel; Daniel, Geoffrey; Jellison, Jody; Yoshida, Makoto; Groom, Leslie; Pingali, Sai Venkatesh; O’Neill, Hugh (2017) Modification of the nanostructure of lignocellulose cell walls via a non-enzymatic lignocellulose deconstruction system in brown rot wood-decay fungi. Biotechnology for Biofuels 10:179. https://doi.org/10.1186/s13068-017-0865-2
- Ma, Ding; Lee, Christoper M; Chen, Yizhu; Mehta, Nikhil; Kim, Seong H; Liu, Zhiwen (2017) Vibrational sum frequency generation digital holography. Applied Physics Letters 110: 251601. https://doi.org/10.1063/1.4986451 Selected as an Editor's Pick!
- Zhang, Tian; Vavylonis, Dimitrios; Durachko, Daniel M; Cosgrove, Daniel J (2017) Nanoscale Movements of Cellulose Microfibrils in Primary Cell Walls. Nature Plants 3: 17056 http://dx.doi.org/10.1038/nplants.2017.56
- Barnes, William J; and Anderson Charles T (2017) Acetyl Bromide Soluble Lignin (ABSL) Assay for Total Lignin Quantification from Plant Biomass. Bio-protocol 2017 7(5): e2149. doi 10.21769/BioProtoc.2149
- Makarem, Mohamadamin; Sawada, Daisuke; O'Neill, Hugh M; Lee, Christopher M; Kafle, Kabindra; Park, Yong Bum; Mittal, Ashutosh; Kim, Seong H (2017) Dependence of Sum Frequency Generation (SFG) Spectral Features on the Mesoscale Arrangement of SFG-Active Crystalline Domains Interspersed in SFG-Inactive Matrix: A Case Study with Cellulose in Uniaxially Aligned Control Samples and Alkali-Treated Secondary Cell Walls in Plants. J. Phys. Chem. C 121(18): 10249-10257. doi 10.1021/acs.jpcc.7b02770
- Chae, Inseok; Ahmed, Saad; Atitallah, Hassene Ben; Luo, Jiawei; Wang, Qing; Ounaies, Zoubeida; Kim, Seong H (2017) Vibrational Sum Frequency Generation (SFG) Analysis of Ferroelectric Response of PVDF-Based Copolymer and Terpolymer. Macromolecules 50(7): 2838-2844. http://dx.doi.org/10.1021/acs.macromol.7b00188
- Kim, Hee Jin; Lee, Christopher M; Dazen, Kevin; Delhom, Christopher D; Liu, Yongliang; Rodgers, James E; French, Alfred D; Kim, Seong H (2017) Comparative physical and chemical analyses of cotton fibers from two near isogenic upland lines differing in fiber wall thickness. Cellulose 24: 2385–2401. DOI 10.1007/s10570-017-1282-1
- Zamil, M Shafayet; Yi, Hojae; Puri, Virendra M (2017) A multiscale FEA framework for bridging cell-wall to tissue-scale mechanical properties: the contributions of middle lamella interface and cell shape. Journal of Materials Science 52 (13): 7947–7968. doi 10.1007/s10853-017-0999-4
- Marek, Antonin; Voinov, Maxim A; Smirnov, Alex I (2017) Spin Probe Multi-Frequency EPR Study of Unprocessed Cotton Fibers. Cell Biochem Biophys 75: 211. DOI 10.1007/s12013-017-0787-4
- Xiao, Chaowen; Barnes, William J; Zamil, M Shafayet; Yi, Hojae; Puri, Virendra M; Anderson, Charles T (2017) Activation tagging of Arabidopsis POLYGALACTURONASE INVOLVED IN EXPANSION2 promotes hypocotyl elongation, leaf expansion, stem lignification, mechanical stiffening, and lodging. Plant Journal 89: 1159–1173 . doi http://onlinelibrary.wiley.com/doi/10.1111/tpj.13453/epdf
- Basu, Snehasish, Omadjela, Okako; Zimmer, Jochen; Catchmark, Jeffrey M (2017) Impact of plant matrix polysaccharides on cellulose produced by surface-tethered cellulose synthases. Carbohydrate Polymers 162 (2017) 93–99 doi 10.1016/j.carbpol.2017.01.005
- Lee, Christopher M; Chen, Xing; Weiss, Philip A; Jensen, Lasse; Kim, Seong H (2017) Quantum Mechanical Calculations of Vibrational Sum-Frequency-Generation (SFG) Spectra of Cellulose: Dependence of the CH and OH Peak Intensity on the Polarity of Cellulose Chains within the SFG Coherence Domain. The Journal of Physical Chemistry Letters 8: 55-60. doi 10.1021/acs.jpclett.6b02624
- Vandavasi, Vandavasi Gopal; and O’Neill, Hugh (2016) Heterologous Expression and Purification of Catalytic Domain of CESA1 from Arabidopsis. Bio-protocol 6(20): e1965. doi 10.21769/BioProtoc.1965
- Wang, Bo; McClosky, Daniel D; Anderson, Charles T; Gong, Chen (2016) Synthesis of a suite of click-compatible sugar analogs for probing carbohydrate metabolism. Carbohydrate Research 433: 54-62. doi 10.1016/j.carres.2016.07.012
- Xiao, Chaowen; and Anderson, Charles T (2016) Interconnections between cell wall polymers, wall mechanics, and cortical microtubules: Teasing out causes and consequences. Article Addendum in Plant Signaling & Behavior 11(9): e1215396. doi 10.1080/15592324.2016.1215396
- Wang, Tuo; Chen, Yuning; Tabuchi, Akira; Hong, Mei; and Cosgrove, Daniel (2016) The Target of β-Expansin EXPB1 in Maize Cell Walls from Binding and Solid-State NMR Studies. Plant Physiology 172(4): 2107-2119. doi 10.1104/pp.16.01311
- Pandey, Jyotsna L.; Kiemle, Sarah N; Richard, Tom L.; Zhu, Yimin; Cosgrove, Daniel; and Anderson, Charles T. (2016) Investigating Biochemical and Developmental Dependencies of Lignification with a Click-Compatible Monolignol Analog in Arabidopsis thaliana Stems. Front. Plant Sci.7:1309. doi 10.3389/fpls.2016.01309
- Li, Shundai; Bashline, Logan; Zheng, Yunzhen; Xin, Xiaoran; Huang, Shixin; Kong, Zhaosheng; Kim, Seong H; Cosgrove, Daniel; and Gu, Ying (2016) Cellulose synthase complexes act in a concerted fashion to synthesize highly aggregated cellulose in secondary cell walls of plants. Proc. Natl. Acad. Sci. U. S. A. 113(40): 11348-11353 doi 10.1073/pnas.1613273113
- Purushotham, Pallinti; Cho, Sung Hyun; Díaz-Moreno, Sara M; Kumar, Manish; Nixon, B. Tracy; Bulone, Vincent; and Zimmer, Jochen (2016) A single heterologously expressed plant cellulose synthase isoform is sufficient for cellulose microfibril formation in vitro. Proc. Natl. Acad. Sci. U. S. A. 113(4): 11360-11365 doi 10.1073/pnas.1606210113
- Wang, Tuo; Phyo, Pyae; and Hong, Mei (2016) Multidimensional solid-state NMR spectroscopy of plant cell walls. Solid State Nucl. Magn. Reson. 78: 56-63. doi 10.1016/j.ssnmr.2016.08.001
- Ogawa, Yu; Lee, Christopher M; Nishiyama, Yoshiharu; and Kim, Seong H (2016) Absence of Sum Frequency Generation in Support of Orthorhombic Symmetry of α-Chitin. Macromolecules 49(18): 7025-7031. doi 10.1021/acs.macromol.6b01583
- Haigler, Candace H.; Davis, Jonathan K.; Slabaugh, Erin; and Kubicki, James D (2016) Biosynthesis and assembly of cellulose. Molecular Cell Biology of the Growth and Differentiation of Plant Cells (pp. 125-143). doi 10.1201/b20316
- Nixon, B. Tracy; Mansouri, Katayoun; Singh, Abhishek; Du, Juan; Davis, Jonathan K.; Lee, Jung-Goo; Slabaugh, Erin; Vandavasi, Venu Gopal; O'Neill, Hugh; Roberts, Eric M.; Roberts, Alison W.; Yingling, Yaroslava G.; and Haigler, Candace H. (2016) Comparative Structural and Computational Analysis Supports Eighteen Cellulose Synthases in the Plant Cellulose Synthesis Complex. Sci. Rep. 6: 28696. doi 10.1038/srep28696
- Du, Juan; Vepachedu, Venkata; Cho, Sung Hyun; Kumar, Manish; and Nixon, B. Tracy (2016) Structure of the Cellulose Synthase Complex of Gluconacetobacter hansenii at 23.4 Å Resolution. PLoS One 11(5): e0155886. doi 10.1371/journal.pone.0155886
- Wang, Tuo; Yang, Hui; Kubicki, James D; and Hong, Mei (2016) Cellulose Structural Polymorphism in Plant Primary Cell Walls Investigated by High-Field 2D Solid-State NMR spectroscopy and Density Functional Theory Calculations. Biomacromolecules 17: 2210-2222. doi 10.1021/acs.biomac.6b00441
- Slabaugh, Erin; Scavuzzo-Duggan, Tess R; Chaves, Arielle M; Wilson, Liza; Wilson, Carmen; Davis, Jonathan K; Cosgrove, Daniel J; Anderson, Charles T; Roberts, Alison W; and Haigler, Candace H (2016) The valine and lysine residues in the conserved FxVTxK motif are important for the function of phylogenetically distant plant cellulose synthases. Glycobiology 26(5): 509-519. doi 10.1093/glycob/cwv118
- Tran, Mai L; and Roberts, Alison W (2015) Cellulose synthase (CESA) gene expression profiling of Physcomitrella patens. Plant Biol. (Berlin, Ger.) 18(3): 363-368. doi 10.1111/plb.12416
- McClosky, Daniel D; Wang, Bo; Chen, Gong; and Anderson, Charles T. (2016) The click-compatible sugar 6-deoxy-alkynyl glucose metabolically incorporates into Arabidopsis root hair tips and arrests their growth. Phytochemistry (Elsevier) 123: 16-24. doi 10.1016/j.phytochem.2016.01.007
- Berry, Elizabeth A; Tran, Mai L; Dimos, Christos S; Budziszek, Michael J; Scavuzzo-Duggan, Tess R; and Roberts, Alison W. (2016) Immuno and affinity cytochemical analysis of cell wall composition in the moss Physcomitrella patens. Front. Plant Sci. 7: 248. doi 10.3389/fpls.2016.00248
 Rui, Yue; and Anderson, Charles T (2016) Functional analysis of cellulose and xyloglucan in the walls of stomatal guard cells of Arabidopsis thaliana. Plant Physiol. 170: 1398-1419. doi 10.1104/pp.15.01066
Rui, Yue; and Anderson, Charles T (2016) Functional analysis of cellulose and xyloglucan in the walls of stomatal guard cells of Arabidopsis thaliana. Plant Physiol. 170: 1398-1419. doi 10.1104/pp.15.01066- Sethaphong, Latsavongsakda; Davis, Jonathan K; Slabaugh, Erin; Singh, Abhishek; Haigler, Candace H; and Yingling, Yaroslava G (2016) Prediction of the structures of the plant-specific regions of vascular plant cellulose synthases and correlated functional analysis. Cellulose 23(1): 145-161. doi 10.1007/s10570-015-0789-6 In addition, their image was chosen for the cover of the February 2016 issue of Cellulose
- Nagachar, Nivedita; and McManus, John B (2016) Microbial Cellulose Synthesis. Microbial Factories: Biofuels, Waste Treatment 1: 203-216. doi 10.1007/978-81-322-2598-0
- Basu, Snehasish; Omadjela, Okako; Gaddes, David; Tadigadapa, Srinivas; Zimmer, Jochen; and Catchmark, Jeffrey M (2016) Cellulose Microfibril Formation by Surface-Tethered Cellulose Synthase Enzymes. ACS Nano 10: 1896-1907. doi 10.1021/acsnano.5b05648
 Cosgrove, Daniel (2016) Catalysts of plant cell wall loosening (2016) F1000Research 5: 119. doi 10.12688/f1000research.7180.1
Cosgrove, Daniel (2016) Catalysts of plant cell wall loosening (2016) F1000Research 5: 119. doi 10.12688/f1000research.7180.1- Zhang, Tian; Zheng, Yunzhen; and Cosgrove, Daniel J (2016) Spatial Organization of Cellulose Microfibrils and Matrix Polysaccharides in Primary Plant Cell Walls as Imaged by Multi-Channel Atomic Force Microscopy. Plant J. 85(2): 179-192. doi 10.1111/tpj.13102 In addition, their image was chosen for the cover of the January 2016 issue of The Plant Journal.
- Lee, Christopher M; Kafle, Kabindra; Huang, Shixin; and Kim, Seong H (2016) Multimodal Broadband Vibrational Sum Frequency Generation (MM-BB-V-SFG) Spectrometer and Microscope. J. Phys. Chem. B 120(1): 102-116. doi 10.1021/acs.jpcb.5b10290
- Cosgrove, Daniel J (2016) Plant cell wall extensibility: connecting plant cell growth with cell wall structure, mechanics, and the action of wallmodifying enzymes. J. Exp. Bot. 67(2): 463-476 (2016). doi 10.1093/jxb/erv511
- Anderson, Charles T (2016) We be jammin’: an update on pectin biosynthesis, trafficking and dynamics. J. Exp. Bot. 67(2): 495-502. doi 10.1093/jxb/erv501
- Wang, Tuo; and Hong, Mei (2016) Solid-state NMR investigations of cellulose structure and interactions with matrix polysaccharides in plant primary cell walls. J. Exp. Bot. 67(2): 503-514. doi 10.1093/jxb/erv416
 Vandavasi, Venu Gopal; Putnam, Daniel K; Zhang, Qiu; Petridis, Loukas; Heller, William T; Nixon, B. Tracy; Haigler, Candace H; Kalluri, Udaya; Coates, Leighton; Langan, Paul; Smith, Jeremy C; Meiler, Jens; and O'Neill, Hugh A (2016) Structural Study of CESA1 catalytic domain of Arabidopsis thaliana Cellulose Synthesis Complex: Evidence for CESA trimers. Plant Physiol. 170: 123-135. doi 10.1104/pp.15.01356 In addition, their image was chosen for the cover of the January 2016 issue of Plant Physiology.
Vandavasi, Venu Gopal; Putnam, Daniel K; Zhang, Qiu; Petridis, Loukas; Heller, William T; Nixon, B. Tracy; Haigler, Candace H; Kalluri, Udaya; Coates, Leighton; Langan, Paul; Smith, Jeremy C; Meiler, Jens; and O'Neill, Hugh A (2016) Structural Study of CESA1 catalytic domain of Arabidopsis thaliana Cellulose Synthesis Complex: Evidence for CESA trimers. Plant Physiol. 170: 123-135. doi 10.1104/pp.15.01356 In addition, their image was chosen for the cover of the January 2016 issue of Plant Physiology.- McManus, John B; Deng, Ying; Nagachar, Nivedita; Kao, Teh-hui; and Tien, Ming (2016) AcsA–AcsB: The core of the cellulose synthase complex from Gluconacetobacter hansenii ATCC23769. Enzyme Microb. Technol. 82: 58-65. doi 10.1016/j.enzmictec.2015.08.015
- Xiao, Chaowen; Zhang, Tian; Zheng, Yunzhen; Cosgrove, Daniel J; and Anderson, Charles T (2016) Xyloglucan deficiency disrupts microtubule stability and cellulose biosynthesis in Arabidopsis, altering cell growth and morphogenesis. Plant Physiol. 170(1): 234-249. doi 10.1104/pp.15.01395
- Dumont, Marie; Lehner, Arnaud; Bardor, Muriel; Burel, Carole; Vauzeilles, Boris; Lerouxel, Olivier; Anderson, Charles T; Mollet, Jean-Claude; and Lerouge, Patrice (2015) Inhibition of fucosylation of cell wall components by 2-fluoro 2-deoxy-L-fucose induces defects in root cell elongation. Plant J. 84(6): 1137-1151. doi 10.1111/tpj.13071
- Lee, Christopher M; Kubicki, James D; Fan, Bingxin; Zhong, Linghao; Jarvis, Michael C; and Kim, Seong H (2015) Hydrogen-Bonding Network and OH Stretch Vibration of Cellulose: Comparison of Computational Modeling with Polarized IR and SFG Spectra. J. Phys. Chem. B 119(49): 15138-15149. doi 10.1021/acs.jpcb.5b08015
- Li, Shundai; Lei, Lei; Yingling, Yaroslava G; and Gu, Ying (2015) Microtubules and cellulose biosynthesis: the emergence of new players. Curr. Opin. Plant Biol. 28: 76-82. doi 10.1016/j.pbi.2015.09.002
 Park, Yong Bum; Kafle, Kabindra; Lee, Christopher M; Cosgrove, Daniel J; and Kim, Seong H (2015) Does cellulose II exist in native alga cell walls? Cellulose structure of Derbesia cell walls studied with SFG, IR and XRD. Cellulose 22(6): 3531-3540. doi 10.1007/s10570-015-0750-8 In addition, their image was chosen for the cover of the December 2015 issue of Cellulose.
Park, Yong Bum; Kafle, Kabindra; Lee, Christopher M; Cosgrove, Daniel J; and Kim, Seong H (2015) Does cellulose II exist in native alga cell walls? Cellulose structure of Derbesia cell walls studied with SFG, IR and XRD. Cellulose 22(6): 3531-3540. doi 10.1007/s10570-015-0750-8 In addition, their image was chosen for the cover of the December 2015 issue of Cellulose.- Erbakan, Mustafa; Curtis, Brandon S; Nixon, B. Tracy; Kumar, Manish; and Curtis, Wayne R (2015) Advancing Rhodobacter sphaeroides as a platform for expression of functional membrane proteins. Protein Expression Purif. 8(7): 1011-1023. doi 10.1016/j.pep.2015.05.012
- Lee, Christopher M; Gu, Jin; Kafle, Kabindra; Catchmark, Jeffrey M; and Kim, Seong H (2015) Cellulose produced by Gluconacetobacter xylinus strains ATCC 53524 and ATCC 23768: pellicle formation, post-synthesis aggregation and fiber density. Carbohydr. Polym. 133: 270-276. doi 10.1016/j.carbpol.2015.06.091
- Bashline, Logan; Li, Shundai; Zhu, Xiaoyu; and Gu, Ying (2015) The TWD40-2 protein and the AP2 complex cooperate in the clathrin-mediated endocytosis of cellulose synthase to regulate cellulose biosynthesis. Proc. Natl. Acad. Sci. U.S.A. 112(41): 12870-12875. doi 10.1073/pnas.1509292112
- Kafle, Kabindra; Shin, H; Lee, Christopher M; Park, Sunkyu; and Kim, Seong H (2015) Progressive structural changes of Avicel, bleached softwood, and bacterial cellulose during enzymatic hydrolysis. Sci. Rep. 5: 15102. doi10.1038/srep15102
- Lei, Lei; Singh, Abhishek; Bashline, Logan; Li, Shundai; Yingling, Yaroslava G; and Gu, Ying (2015) CELLULOSE SYNTHASE INTERACTIVE1 Is Required for Fast Recycling of Cellulose Synthase Complexes to the Plasma Membrane in Arabidopsis. Plant Cell 27(10): 2926-2940. doi 10.1105/tpc.15.00442
- Zamil, M. Shafayet; Yi, Hojae; and Puri, Virendra M (2015)The mechanical properties of plant cell walls soft material at the subcellular scale: the implications of water and of the intercellular boundaries. J. Mater. Sci. 50(20): 6608-6623. doi 10.1007/s10853-015-9204-9
- Bi, Yunchen; Hubbard, Caitlin; Purushotham, Pallinti; and Zimmer, Jochen (2015) Insights into the structure and function of membrane-integrated processive glycosyltransferases. Curr. Opin. Struct. Biol. 34: 78-86. doi 10.1016/j.sbi.2015.07.008
- Cho, Sung Hyun; Du, Juan; Sines, Ian; Poosarla, Venkata Giridhar; Vepachedu, Venkata; Kafle, Kabindra; Park, Yong Bum; Kim, Seong H; Kumar, Manish; and Nixon, B. Tracy (2015) In vitro synthesis of cellulose microfibrils by membrane protein from protoplasts of the non-vascular plant Physcomitrella patens. Biochem. J. 470(2): 195-205. doi 10.1042/BJ20141391
- Lee, Christopher M; Dazen, Kevin; Kafle, Kabindra; Moore, Andrew; Johnson, David K; Park, Sunkyu; and Kim, Seong H (2015) Correlations of Apparent Cellulose Crystallinity Determined by XRD, NMR, IR, Raman, and SFG Methods. Adv. Polym. Sci., 1-17. doi 10.1007/12_2015_320
- Erbakan, Mustafa; Curtis, Brandon S; Nixon, B. Tracy; Kumar, Manish; and Curtis, Wayne R (2015) Advancing Rhodobacter sphaeroides as a platform for expression of functional membrane proteins. Protein Expression Purif. 8(7): 1011-1023. doi 10.1016/j.pep.2015.05.012
- Wang, Tuo; Park, Yong Bum; Cosgrove, Daniel; and Hong, Mei (2015) Cellulose-Pectin Spatial Contacts Are Inherent to Never-Dried Arabidopsis thaliana Primary Cell Walls: Evidence from Solid-State NMR. Plant Physiol. 168 (3): 871-884. doi 10.1104/pp.15.00665
- Kong, Zhaosheng; Ioki, Motohide; Braybrook, Siobhan; Li, Shundai; Ye, Zheng-Ha; Lee, Yuh-Ru Julie; Hotta, Takashi; Chang, Anny; Tian, Juan; Wang, Guangda; and Liu, Bo (2015) Kinesin-4 functions in vesicular transport on cortical microtubules and regulates cell wall mechanics during cell elongation in plants. Mol. Plant 8(7): 1011-1023. doi 10.1016/j.molp.2015.01.004
- Scavuzzo-Duggan, Tess R; Chaves, Arielle M; and Roberts, Alison W (2015) A complementation assay for in vivo protein structure/function analysis in Physcomitrella patens (Funariaceae). Appl. Plant Sci. 3(7): 1500023. doi 10.3732/apps.1500023
- McNamara, Joshua T; Morgan, Jacob LW; and Zimmer, Jochen (2015) A Molecular Description of Cellulose Biosynthesis. Annu. Rev. Biochem. 84, 895-921. doi 10.1146/annurev-biochem-060614-033930
- Yang, Hui; Zimmer, Jochen; Yingling, Yaroslava G; and Kubicki, James D (2015) How Cellulose Elongates - a QM/MM Study of the Molecular Mechanism of Cellulose Polymerization in Bacterial CESA. J. Phys. Chem. B 119 (22): 6525–6535. doi 10.1021/acs.jpcb.5b01433
- Pandey, Jyotsna L.; Wang, Bo; Diehl, Brett G.; Richard, Tom L; Chen, Gong; and Anderson, Charles T (2015) A Versatile Click-Compatible Monolignol Probe to Study Lignin Deposition in Plant Cell Walls. PLoS One 10(4): e0121334. doi 10.1371/journal.pone.0121334
- Lee, Christopher M; Kafle, Kabindra; Belias, David; Park, Yong Bum; Glick, Richard E; Haigler, Candace H; and Kim, Seong H (2015) Comprehensive analysis of cellulose content, crystallinity, and lateral packing in Gossypium hirsutum and Gossypium barbadense cotton fibers using sum frequency generation, infrared and Raman spectroscopy, and X-ray diffraction. Cellulose 22 (2): 971-989. doi 10.1007/s10570-014-0535-5
- Nili, Abdolmajid; Yi, Hojae; Crespi, Vincent H; and Puri, Virendra M (2015) Examination of biological hotspot hypothesis of primary cell wall using a computational cell wall network model. Cellulose 22: 1027-1038. doi 10.1007/s10570-015-0568-4
- Johnson, Quentin R; Lindsay, Richard J; Petridis, Loukas; and Shen, Tongye (2015) Investigation of Carbohydrate Recognition via Computer Simulation. Molecules 20(5): 7700-7718. doi 10.3390/molecules20057700
- Kapp, Nikki; Barnes, William J; Richard, Tom L; and Anderson, Charles T (2015) Imaging with the fluorogenic dye Basic Fuchsin reveals subcellular patterning and ecotype variation of lignification in Brachypodium distachyon. J. Exp. Bot. 66 (14): 4295-4304. doi 10.1093/jxb/erv158
- Deng, Ying; Nagachar, Nivedita; Fang, Lin; Luan, Xin; Catchmark, Jeffrey M; Tien, Ming; and Kao, Teh-hui (2015) Isolation and Characterization of Two Cellulose Morphology Mutants of Gluconacetobacter hansenii ATCC23769 Producing Cellulose with Lower Crystallinity. PLoS One, 10 (3), e0119504. doi 10.1371/journal.pone.0119504
- Zhu, Chuanmei; Ganguly, Anindya; Baskin, Tobias I; McClosky, Daniel D; Anderson, Charles T; Foster, Cliff; Meunier, Kristoffer A; Okamoto, Ruth; and Dixit, Ram (2015) The Fragile Fiber1 kinesin contributes to cortical microtubule-mediated trafficking of cell wall components. Plant Physiol. 167: 780-792. doi 10.1104/pp.114.251462
- Park, Yong Bum; and Cosgrove, Daniel (2015) Xyloglucan and its interactions with other components of the growing cell wall. Plant Cell Physiol. 56 (2): 180-194. doi 10.1093/pcp/pcu204
- Fan, Bingxin; and Maranas, Janna K (2015) Coarse-Grained Simulation of Cellulose Iβ with Application to Long Fibrils. Cellulose 22: 31-44. doi 10.1007/s10570-014-0481-2
- Bashline, Logan; and Gu, Ying (2015) Using the Split-Ubiquitin yeast two-hybrid systems to test protein-protein interactions of transmembrane proteins. Methods Mol. Biol. (N. Y., NY, U. S.) 1242: 143-158. doi 10.1007/978-1-4939-1902-4_13
- Kim, Keekyoung; Yi, Hojae; Zamil, M Shafayet; Haque, M. Aman; and Puri, Virendra M (2015) Multiscale stress-strain characterization of outer onion epidermal peel tissue in wet and dry states. Am. J. Bot. 102: 12-20. doi 10.3732/ajb.1400273
- Fang, Lin; and Catchmark, Jeffrey M (2015) Characterization of cellulose and other exopolysaccharides produced from Gluconacetobacter strains. Carbohydr. Polym. 115: 663-669. doi 10.1016/j.carbpol.2014.09.028
- Xi, Xiaoning; Kim, Seong H; and Tittmann, Bernhard (2015) Atomic force microscopy based nanoindentation study of onion abaxial epidermis walls in aqueous environment. J. Appl. Phys. 117: 024703. [doi 10.1063/1.4906094
- Fang, Lin; and Catchmark, Jeffrey M (2014) Characterization of water-soluble exopolysaccharides from Gluconacetobacter xylinus and their impacts on bacterial cellulose crystallization and ribbon assembly. Celulose 21: 3965-3978. doi 10.1007/s10570-014-0443-8
- Fang, Lin; and Catchmark, Jeffrey M (2014) Structure characterization of native cellulose during dehydration and rehydration. Cellulose 21: 3951-3963. doi 10.1007/s10570-014-0435-8
- Shklyaev, Oleg E; Kubicki, James D; Watts, Heath D; and Crespi, Vincent H (2014) Constraints on Iβ cellulose twist from DFT calculations of 13C NMR chemical shifts. Cellulose 21: 3979-3991. doi 10.1007/s10570-014-0448-3
- Cosgrove, Daniel (2014) Re-constructing our models of cellulose and primary cell wall assembly. Curr. Opin. Plant Biol. 22: 122-131. doi 10.1016/j.pbi.2014.11.001
- Hill,Joseph Lee; Hammudi, Mustafa B; Tien, Ming (2014) The Arabidopsis Cellulose Synthase Complex: A Proposed Hexamer of CESA Trimers in an Equimolar Stoichiometry. Plant Cell 26: 4834-4842. doi 10.1105/tpc.114.131193
- Xiao, Chaowen; and Anderson, Charles T (2014) Activation Tag Screening for Cell Expansion Genes in Arabidopsis thaliana. Methods Mol. Biol. (N. Y.) 1242: 159-171. doi 10.1007/978-1-4939-1902-4_14
- Bukowski, Natalie; Pandey, Jyotsna L; Doyle, Lucas; Richard, Tom L; Anderson, Charles T; Zhu, Yimin (2014) Development of a Clickable Designer Monolignol for Interrogation of Lignification in Plant Cell Walls. Bioconjugate Chem. 25 (12): 2189–2196. doi 10.1021/bc500411u
- Kafle, Kabindra; Greeson, Kenneth; Lee, Christopher M; Kim, Seong H (2014) Cellulose polymorphs and physical properties of cotton fabrics processed with commercial textile mills for mercerization and liquid ammonia treatments. Text. Res. J. 84: 1692-1699. doi 10.1177/0040517514527379
- Petridis, Loukas; O'Neill, Hugh M; Johnsen, Mariah; Fan, Bingxin; Schulze, Roland K; Mamontov, E; Maranas, Janna K; Langan, Paul; and Smith, Jeremy C (2014) Hydration Control of the Mechanical and Dynamical Properties of Cellulose. Biomacromolecules 15: 4152–4159. doi 10.1021/bm5011849
- Slabaugh, Erin; Sethaphong, Latsavongsakda; Xiao, Chaowen; Amick, Joshua; Anderson, Charles T; Haigler, Candace H; and Yingling, Yaroslava G (2014) Computational and genetic evidence that different structural conformations of a non-catalytic region affect the function of plant cellulose synthase. J. Exp. Bot. 65: 6645-6653. doi 10.1093/jxb/eru383
- Tittmann, Bernhard; and Xi, Xiaoning (2014) Imaging and quantitative data acquisition of biological cell walls with Atomic Force Microscopy and Scanning Acoustic Microscopy Microscopy Book Series #6: “Microscopy: advances in scientific research and education” 2: 161-172. doi 10.2172/1158577
- Kafle, Kabindra; Shi, Rui; Lee, Christopher M; Mittal, Ashutosh; Park, Yong Bum; Sun, Ying-Hsuan; Park, Sunkyu; Chiang, Vincent; and Kim, Seong H (2014) Vibrational sum-frequency-generation (SFG) spectroscopy study of the structural assembly of cellulose microfibrils in reaction woods. Cellulose 21: 2219-2231. doi 10.1007/s10570-014-0322-3
- White, Paul B; Wang, Tuo; Park, Yong Bum; Cosgrove, Daniel; and Hong, Mei (2014) Water – Polysaccharide Interactions in the Primary Cell Wall of Arabidopsis thaliana from Polarization Transfer Solid-State NMR. J. Am. Chem. Soc. 136: 10399-10409. doi 10.1021/ja504108h
- Lei, Lei; Zhang, Tian; Strasser, Richard; Lee, Christopher M; Gonneau, Martine; Mach, Lukas; Vernhettes, Samantha; Kim, Seong H; Cosgrove, Daniel J; Li, Shundai; and Gu, Ying (2014)The jiaoyao1 Mutant Is an Allele of korrigan1 That Abolishes Endoglucanase Activity and Affects the Organization of Both Cellulose Microfibrils and Microtubules in Arabidopsis. Plant Cell 26: 2601–2616. doi 10.1105/tpc.114.126193
- Diehl, Brett G; Watts, Heath D; Kubicki, James D; Regner, Matthew R; Ralph, John; Brown, Nicole Robitaille (2014) Towards lignin-protein crosslinking: amino acid adducts of a lignin model quinone methide. Cellulose 21: 1395-1407. doi 10.1007/s10570-014-0181-y
- Park, Yong Bum; Lee, Christopher M; Kafle, Kabindra; Park, Sunkyu; Cosgrove, Daniel J; and Kim, Seong H (2014) Effects of Plant Cell Wall Matrix Polysaccharides on Bacterial Cellulose Structure Studied with Vibrational Sum Frequency Generation Spectroscopy and X‑ray Diffraction. Biomacromolecules 15: 2718-2724. doi 10.1021/bm500567v
- Tittmann, Bernhard; Maghsoudy-Louyeh, Sahar; Kim, Jeong; and Xi, Xiaoning (2014) Imaging of Biological Nano-Composite Plant Cell Wall at the Micro- and Nano-Scales. Nondestr. Charact. Mater. XIII, Proc. Int. Symp. 13th. 19: 4.
- Zamil, M. Shafayet; Yi, Hojae; and Puri, Virendra M (2014) Mechanical characterization of onion outer epidermal middle lamella under tensile loading. Am. J. Bot. 101(5). doi 10.3732/ajb.1300416
- Lee, Christopher M; Kafle, Kabindra; Park, Yong Bum; and Kim, Seong H (2014) Probing crystal structure and mesoscale assembly of cellulose microfibrils in plant cell walls, tunicate tests, and bacterial films using vibrational Sum Frequency Generation (SFG) spectroscopy. Phys. Chem. Chem. Phys. 16: 10844-10853. doi 10.1039/C4CP00515E
- Kubicki, James D; Watts, Heath D; Zhao, Zhen; and Zhong, Linghao (2014) Quantum Mechanical Calculations on Cellulose-Water Interactions: Structures, Energetics, Vibrational Frequencies and NMR Chemical Shifts for Surfaces of Iα and Iβ Cellulose. Cellulose 21: 909-926. doi 10.1007/s10570-013-0029-x
- Zhang, Tian; Mahgsoudy-Louyeh, Sahar; Tittmann, Bernhard; and Cosgrove, Daniel (2014) Visualization of the nanoscale pattern of recently-deposited cellulose microfibrils and matrix materials in never-dried primary walls of the onion epidermis. Cellulose 21: 853-862. doi 10.1007/s10570-013-9996-1
- Haigler, Candace H; Grimson, Mark J; Gervais, Julien; Le Moigne, Nicolas; Ho¨fte, Herman; Monasse, Bernard; and Navard, Patrick (2014) Molecular Modeling and Imaging of Initial Stages of Cellulose Fibril Assembly: Evidence for a Disordered Intermediate Stage. PLoS One 9(4), e93981. Open access: http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0093981
- Kafle, Kabindra; Xi, Xiaoning; Lee, Christopher M; Tittmann, Bernhard; Cosgrove, Daniel J; Park, Yong Bum; and Kim, Seong H (2014) Cellulose microfibril orientation in onion (Allium cepa L.) epidermis studied by atomic force microscopy (AFM) and vibrational sum frequency generation (SFG) spectroscopy. Cellulose 21: 1075-1086. doi 10.1007/s10570-013-0121-2
- Zhao, Zhen; Crespi, Vincent H; Kubicki, James D; Cosgrove, Daniel J; and Zhong, Linghao (2014) Molecular dynamics simulation study of xyloglucan adsorption on cellulose surfaces: effects of surface hydrophobicity and side-chain variation. Cellulose 21: 1025-1039. doi 10.1007/s10570-013-0041-1
- McCarthy, Thomas W; Der, Joshua P; Honaas, Loren A; dePamphilis, Claude W; and Anderson, Charles T (2014) Phylogenetic analysis of pectin-related gene families in Physcomitrella patens and nine other plant species yields evolutionary insights into cell walls. BMC Plant Biol. 14: 79. doi 10.1186/1471-2229-14-79
- Xiao, Chaowen; Somerville, Chris R; Anderson, Charles T (2014) POLYGALACTURONASE INVOLVED IN EXPANSION1 Functions in Cell Elongation and Flower Development in Arabidopsis. Plant Cell 26: 1018-1035. doi 10.1105/tpc.114.123968
- Kiemle, Sarah N; Zhang, Xiao; Esker, Alan R; Toriz, Guillermo; Gatenholm, Paul; and Cosgrove, Daniel J (2014) Role of (1,3)(1,4)-β-Glucan in Cell Walls: Interaction with Cellulose Biomacromolecules 15(5): 1727-1736. doi 10.1021/bm5001247
- Slabaugh, Erin; Davis, Jonathan K; Haigler, Candace H; Yingling, Yaroslava G; and Zimmer, Jochen (2014) Cellulose synthases: new insights from crystallography and modeling. Trends Plant Sci. 19: 99-106. doi 10.1016/j.tplants.2013.09.009
- Yi, Hojae; and Puri, Virendra M (2014) Contributions of the mechanical properties of major structural polysaccharides to the stiffness of a primary cell wall network model. Am. J. Bot. 101: 1-11. doi 10.3732/ajb.1300315
- Gu,Jin; and Catchmark, Jeffrey M (2014) Roles of xyloglucan and pectin on the mechanical properties of bacterial cellulose composite films. Cellulose 21: 275-289. doi 10.1007/s10570-013-0115-0
- Wang, Chao; and Esker, Alan R (2014) Nanocrystalline chitin thin films. Carbohydr. Polym. 102: 151-158. doi 10.1016/j.carbpol.2013.10.103
- Watts, Heath; Mohamed, Mohamed Naseer Ali; and Kubicki, James D (2014) A DFT study of vibrational frequencies and ¹³C NMR chemical shifts of model cellulosic fragments as a function of size. Cellulose 21 (1): 53-70. doi 10.1007/s10570-013-0128-8
- Kong, Lingyan; Lee, Christopher M; Kim, Seong H; and Ziegler, Gregory R (2013) Characterization of starch polymorphic structures using vibrational sum frequency generation (SFG) spectroscopy J. Phys. Chem. B 118 (7): 1775–1783. doi 10.1021/jp411130n
- Deng, Ying; Nagachar, Nivedita; Xiao, Chaowen; Tien, Ming; and Kao, Teh-hui (2013) Identification and characterization of non-cellulose-producing mutants of Gluconacetobacter hansenii generated by Tn5 transposon mutagenesis. J. Bacteriol. 195: 5072-5083. doi 10.1128/JB.00767-13
- Cong, Fang; Diehl, Brett G; Hill, Joseph Lee; Brown, Nicole Robitaille; and Tien, Ming (2013) Covalent bond formation between amino acids and lignin: Cross coupling between proteins and lignin. Phytochemistry 96: 449-456. doi 10.1016/j.phytochem.2013.09.012
- Xi, Xiaoning; Li, Xin; Miyasaka, C; Kropf, Matthew; and Tittmann, Bernhard (2013) High frequency scanning acoustic microscopy as diagnostic tool in tissue science. J. Biotechnol. Biomater. 3(3): 1000160. doi 10.4172/2155-952X.1000160
- Omadjela, Okako; Narahari, Adishesh; Strumillo, Joanna; Mélida, Hugo; Mazur, Olga; Bulone, Vincent; and Zimmer, Jochen (2013) BcsA and BcsB form the catalytically active core of bacterial cellulose synthase sufficient for in vitro cellulose synthesis. Proc. Natl. Acad. Sci. U. S. A. 110: 17856-17861. doi 10.1073/pnas.1314063110
- Park, Yong Bum; Lee, Christopher M; Koo, Bon-Wook; Park, Sunkyu; Cosgrove, Daniel; and Kim, Seong H (2013) Monitoring meso-scale ordering of cellulose in intact plant cell walls using sum frequency generation (SFG) spectroscopy. Plant Physiol. 163: 907-913. doi 10.1104/pp.113.225235
- Kim, Seong H; Lee, Christopher M; and Kafle, Kabindra (2013) Characterization of crystalline cellulose in biomass: Basic principles, applications, and limitations of XRD, NMR, IR, Raman, and SFG. Korean J. Chem. Eng. 30: 2127-2141. doi 10.1007/s11814-013-0162-0
- Wang, Tuo; Park, Yong Bum; Caporini, Marc A; Rosay, Melanie; Zhong, Linghao; Cosgrove, Daniel; and Hong, Mei (2013) Sensitivity-enhanced solid-state NMR detection of expansin’s target in plant cell walls. Proc. Natl. Acad. Sci. U.S.A. 110: 16444–16449. doi 10.1073/pnas.1316290110
- Bashline, Logan; Li, Shundai; Anderson, Charles T; Lei, Lei; and Gu, Ying (2013) The Endocytosis of Cellulose Synthase in Arabidopsis Is Dependent on μ2, a Clathrin-Mediated Endocytosis Adaptin. Plant Physiology 163: 150-160. Open access: www.plantphysiol.org/cgi/doi/10.1104/pp.113.221234
- Wang, Chao; Qian, Chen; Roman, Maren; Glasser, Wolfgang; and Esker, Alan R (2013) Surface-Initiated Dehydrogenative Polymerization of Monolignols: A Quartz Crystal Microbalance with Dissipation Monitoring and Atomic Force Microscopy Study. Biomacromolecules 14: 3964−3972. doi 10.1021/bm401084h
- Kubicki, James D; Mohamed, Mohamed Naseer Ali; and Watts, Heath D (2013) Quantum mechanical modeling of the structures, energetics and spectral properties of Iα and Iβ cellulose. Cellulose 20: 9-23. doi 10.1007/s10570-012-9838-6
- Handakumbura, Pubudu P; Matos, Dominick A; Osmont, Karen S; Harrington, Michael J; Heo, Kyuyoung; Kafle, Kabindra; Kim, Seong H; Baskin, Tobias I; and Hazen, Samuel P (2013) Perturbation of Brachypodium distachyon CELLULOSE SYNTHASE A4 or 7 results in abnormal cell walls. BMC Plant Biol. 13: 131. doi 10.1186/1471-2229-13-131
- Wang, Chao; Kittle, Joshua D; Qian, Chen; Roman, Maren; Esker, Alan R (2013) Chitinase Activity on Amorphous Chitin Thin Films: A Quartz CrystalMicrobalance with Dissipation Monitoring and Atomic Force Microscopy Study. BioMacromolecules 14: 2622-2628. doi 10.1021/bm4004833
- Gu, Jin; Catchmark Jeffrey (2013) The impact of cellulose structure on binding interactions with hemicellulose and pectin. Cellulose. doi 10.1007/s10570-013-9965-8
- Schultink, A; Cheng, K; Park, Yong Bum,;Cosgrove, Daniel J; Pauly, Markus (2013) The identification of two arabinosyltransferases from tomato reveals functional equivalency of xyloglucan side-chain substituents. Plant Physiology 163: 86-64. Open access: http://dx.doi.org/10.1104/pp.113.221788
- Kim, Seong H; Lee, Christopher M; Kafle, Kabindra; Park, Yong Bum; Xi, Xiaoning (2013) Vibrational Sum Frequency Generation (SFG) Spectroscopic Study of Crystalline Cellulose in Biomass Proc. SPIE 8845: 884508. doi 10.1117/12.2024099
- Maghsoudy-Louyeh, Sahar; Kim, Jeong; Kropf, Matthew; Tittmann, Bernhard (2013) Subsurface image analysis of plant cell wall with atomic force microscopy. J. Adv. Microsc. Res. 8: 100-104. doi 10.1166/jamr.2013.1144
- Zamil, MS; Haque, MA; Yi, Hojae; Puri Virendra M (2013) Characterizing microscale biological samples under tensile loading - Stress-strain behavior of onion outer epidermal cell wall fragment. Am. J. Bot. 100: 1105-1115. doi 10.3732/ajb.1200649
- Lee, Christopher M; Mohamed, Mohamed Naseer Ali; Watts, Heath D; Kubicki, James D; and Kim, Seong H (2013) Sum-Frequency-Generation vibration spectroscopy and density functional theory calculations with dispersion corrections (DFT-D2) for cellulose Iα and Iβ. J. Phys. Chem. B, 117 (22): 6681-6692. doi 10.1021/jp402998s
- Sethaphong, Latsavongsakda; Haigler, Candace H; Kubicki, James D; Zimmer, Jochen; Bonetta, Dario; DeBolt, Seth; Yingling, Yaroslava G (2013) Tertiary model of a plant cellulose synthase. PNAS 110: 7512-7517. doi 10.1073/pnas.1301027110
- Liu, Y; Marshall, J; Qiong, L; Edwards, N; Chen, Gong (2013) Synthesis of novel bivalent mimetic ligands for mannose-6-phosphate receptors. Bioorg. Med. Chem. Lett. 23, 2328–2331. doi 10.1016/j.bmcl.2013.02.068
- Xiao, Chaowen and Anderson, Charles T (2013) Roles of pectin in biomass yield and processing for biofuels. Front. Plant Sci. 4:67, 1-7. doi 10.3389/fpls.2013.00067
- Li, Shundai; Lei, Lei; Gu, Ying (2013) Functional analysis of complexes with mixed primary and secondary cellulose synthases. Plant Signaling Behav. 8(3): e23179. doi 10.4161/psb.23179
- Yi, Hojae; Thakur, S; Sethaphong, Latsavongsakda; Yingling, Yaroslava G (2013) X3DBio2: A Visual Analysis Tool for Biomolecular Structure Comparison. Proc. SPIE: 8654. doi 10.1117/12.2002626
- Kubicki, James D; Mohamed, Mohamed Naseer A; Watts, Heath D (2013)Quantum mechanical modeling of the structures, energetics and spectral properties of Iα and Iβ cellulose. Cellulose 20: 9-23. DOI 10.1007/s10570-012-9838-6. Open access: http://link.springer.com/article/10.1007%2Fs10570-012-9838-6
- Gu, Jin; Catchmark, Jeffrey M; Kaiser, E; Archibald, Douglas (2013) Quantification of cellulose nanowhiskers sulfate esterification levels. Carbohyd Polym. 92(2): 1809–1816. doi 10.1016/j.carbpol.2012.10.078
- Zhao, Zhao; Shklyaev; Oleg E; Nili, Abdolmajid; Mohamed, Mohamed Naseer Ali; Kubicki, James D; Crespi, Vincent H; Zhong, Linghao (2013) Cellulose Microfibril Twist, Mechanics, and Implication for Cellulose Biosynthesis. J. Phys. Chem. A, 117 (12): 2580–2589. doi 10.1021/jp3089929
- Iyer, Prashanti R; Liu, Y-A; Deng, Ying; McManus, John B; Kao, Tei-huo; Tien, Ming(2013) Processing of cellulose synthase (AcsAB) from Gluconacetobacter hansenii 23769. Archives of Biochemistry and Biophysics 529: 92-98. doi 10.1016/j.abb.2012.12.002
- Yi, Hojae; Puri Virendra M (2012) Architecture-based multiscale computational modeling of plant cell wall mechanics to examine the hydrogen-bonding hypothesis of cell wall network structure model. Plant Physiol. 160(3):1281-1292. DOI: 10.1104/pp.112.201228
- Li, Shundai; Gu, Ying (2012) Cellulose biosynthesis in higher plants and the role of the cytoskeleton. eLS. John Wiley & Sons, Ltd: Chichester. DOI: 10.1002/9780470015902.a0023745
- Carroll, A; Mansoori, N; Li, Shundai; Lei, Lei; Vernhettes, Samantha; Visser, RGF; Somerville, Christoper R; Gu, Ying; Trindade, LM (2012) Complexes with mixed primary and secondary cellulose synthases are functional in Arabidopsis thaliana plants. Plant Physiol. 160(2):726-37. DOI: 10.1104/pp.112.199208
- Cosgrove, Daniel J; Jarvis, Michael C (2012) Comparative structure and biomechanics of plant primary and secondary cell walls. Frontiers in Plant Science 3: 204. DOI: 10.3389/fpls.2012.00204
- Baskin, Tobias I; Gu, Ying (2012) Making parallel lines meet: Transferring information from microtubules to extracellular matrix. Cell Adhesion. Migration 6(5): 1-5. DOI: 10.4161/cam.21121
- Roberts, Alison W; Roberts, Eric M; Haigler, Candace H (2012) Moss cell walls: structure and biosynthesis. Front. Plant Sci. 3: 166. DOI: 10.3389/fpls.2012.00166
- Lei, Lei; Li, Shundai; Gu, Ying (2012) Cellulose synthase interactive protein 1 (CSI1) mediates the intimate relationship between cellulose microfibrils and cortical microtubules. Plant Signaling Behavior 7(7): 714-718. DOI: 10.4161/psb.20338
- Watts, Heath D; Archibald, Douglas D; Mohamed, Mohamed Naseer Ali; Kubicki, James D (2012) In search of OH-π interactions between 1-methylimidazole and water using a combined computational quantum chemistry and ATR-FTIR spectroscopy approach. Journal of Molecular Structure 1026: 78-87. DOI: 10.1016/j.molstruc.2012.05.028
- Cheng, Gang; Datta, Supratim; Liu, Zelin; Wang, Chao; Murton, Jaclyn K; Brown, Page A; Jablin, Michael S; Dubey, Manish; Majewski, Jaroslaw; Halbert, Candice E; Browning, James F; Esker, Alan R; Watson, Brian J; Zhang, Haito; Hutcheson, Steven W; Huber, Dale L; Sale, Kenneth L; Simmons, Blake A; and Kent, Michael S (2012) Interactions of endoglucanases with amorphous cellulose films resolved by neutron reflectometry and quartz crystal microbalance with dissipation monitoring. Langmuir 28: 8348-8358. DOI: 10.1021/la300955q
- Wallace, Ian S; Anderson, Charles T (2012) Small molecule probes for plant cell wall polysaccharide imaging. Front. Plant Sci. 3 (89): 1-8. DOI: 10.3389/fpls.2012.00089
- Barnette, Anna L; Lee, Chistopher; Bradley, Laura C; Schreiner, EP; Park, Yung Bum; Shin, H; Cosgrove, Daniel J; Park, Sunkyu; Kim, Seong H (2012) Quantification of crystalline cellulose in lignocellulosic biomass using sum frequency generation (SFG) vibration spectroscopy and comparison with other analytical methods. Carbohydrate Polymers 89(3): 802-809. DOI: 10.1016/j.carbpol.2012.04.014
- Park, Yong Bum Cosgrove, Daniel J (2012) A revised architecture of primary cell walls based on biomechanical changes induced by substrate-specific endoglucanases. Plant Physiology 158: 1933-1943. Open access article: http://www.plantphysiol.org/content/158/4/1933
- Lei, Lei; Li, Shundai; Gu, Ying (2012) Cellulose synthase complexes: composition and regulation. Front. Plant Physiol. 3: 75. DOI: 10.3389/fpls.2012.00075
- Park, Yong Bum; Cosgrove, Daniel J (2012) Changes in cell wall biomechanical properties in the xyloglucan-deficient xxt1/xxt2 mutant of Arabidopsis. Plant Physiology 158: 465-475. DOI: 10.1104/pp.111.189779
- Gu, Jin; Catchmark, Jeffrey M (2012) Impact of hemicelluloses and pectin on sphere-like bacterial cellulose assembly. Carbohydrate Polymers 88: 547-557. DOI: 10.1016/j.carbpol.2011.12.040
- Kittle, Joshua D; Wang, Chao; Qian, Chen; Zhang, Yafen, Zhang, Mingqiang; Roman, Maren; Morris, John R; Moore, Robert B; Esker, Alan E (2012) Ultrathin chitin films for nanocomposites and biosensors. Biomacromolecules 2012 13 (3): 714-718. DOI: 10.1021/bm201631r
- Yi, Hojae; Singh, Abhishek; Yingling, Yaraslava G (2012) X3DBio1: A Visual Analysis Tool for Biomolecular Structure Exploration. Proc, SPIE Visualization and Data Analysis 8294: 82940S-1---82940S-8. DOI: 10.1117/12.906893
- Li, Shundai; Lei, Lei; Somerville, Christopher R; Gu, Ying (2012) Cellulose synthase interactive protein 1 (CSI1) links microtubules and cellulose synthase complexes. Proc Natl Acad Sci U S A 109: 185-190. DOI: 10.1073/pnas.1118560109
- Bashline, Logan; Du, Juan; Gu, Ying (2011) The trafficking and behavior of cellulose synthase and a glimpse of potential cellulose synthesis regulators. Frontiers in Biology 6: 377-383. DOI: 10.1007/s11515-011-1161-3
- Watts, Heath D; Mohamed, Mohamed Naseer Ali; Kubicki, James D (2011) Evaluation of potential reaction mechanisms leading to the formation of coniferyl alcohol α-linkages in lignin: a density functional theory study. Phys Chem Chem Phys 13: 20974-85. DOI: 10.1039/c1cp21906e
- Kittle, Joshua D; Du, Xiaosong; Jiang, F; Qian, Chen; Heinze, T; Roman, Maren; Esker, Alan R (2011) Equilibrium water contents of cellulose films determined via solvent exchange and quartz crystal microbalance with dissipation monitoring. Biomacromolecules 12: 2881-2887. DOI: 10.1021/bm200352q
- Cheng, Gang; Liu, Zelin; Murton, Jaclyn K; Jablin, Michael; Dubey, Manish; Majewski, Jaroslaw; Halbert, Candice; Browning, James; Ankner, John; Akgun, Bulent; Wang, Chao; Esker, Alan R; Sale, Kenneth L; Simmons, Blake A; Kent, Michael S (2011) Neutron reflectometry and QCM-D study of the interaction of cellulases with films of amorphous cellulose. Biomacromolecules 12: 2216-2224. DOI: 10.1021/bm200305u
- Smirnov, Alex I (2011) EPR Studies of Nano Materials. In: Misra SK, editor. Multifrequency Electron Paramagnetic Resonance: Theory and Applications. NY: Wiley. pp. 825-843.
- Barnette, Anna L; Bradley, Laura C; Veres, BD; Schreiner, EP; Park, Yong Bum; Park, Junyeong; Park, Sunkyu; Kim, Seong H (2011) Selective detection of crystalline cellulose in plant cell walls with sum-frequency-generation (SFG) vibration spectroscopy. Biomacromolecules 12: 2434-2439. DOI: 10.1021/bm200518n
- Watts, Heath D; Mohamed, Mohamed Naseer A; Kubicki, James D (2011) Comparison of multistandard and TMS-standard calculated NMR shifts for coniferyl alcohol and application of the multistandard method to lignin dimers. J Phys Chem B 115: 1958-1970. DOI: 10.1021/jp110330q
- Iyer, Prashanti R; Catchmark, Jeffrey; Brown, Nicole R; Tien, Ming (2011) Biochemical localization of a protein involved in synthesis of Gluconacetobacter hansenii cellulose. Cellulose 18: 739-747. DOI: 10.1007/s10570-011-9504-4
- Cosgrove, Daniel J (2011) Measuring In Vitro Extensibility of Growing Plant Cell Walls. Plant Cell Wall: Methods and Protocols 715: 291-303. DOI: 10.1007/978-1-61779-008-9_20
- Iyer, Prashanti R; Geib, SM; Catchmark, Jeffrey; Kao, Teh-hui; Tien Ming (2010) Genome sequence of a cellulose-producing bacterium, Gluconacetobacter hansenii ATCC 23769. J Bacteriol 192: 4256-4257. DOI: 10.1128/JB.00588-10
- Voinov, Maxim A; Smirnov, Alex I (2010) Spin Labels and Spin Probes for Measurements of Local pH and Electrostatics by EPR. In: Chechik V, editor. Cambridge: The Royal Society of Chemistry. pp. 71-106. DOI: 10.1039/9781849730877-00071
- Mohamed, Mohamed Naseer A; Watts, Heath D; Guo, Jing; Catchmark, Jeffrey M; Kubicki, James D (2010) MP2, density functional theory, and molecular mechanical calculations of C-H-π and hydrogen bond interactions in a cellulose-binding module-cellulose model system. Carbohydr Res 345: 1741-1751. DOI: 10.1016/j.carres.2010.05.021








